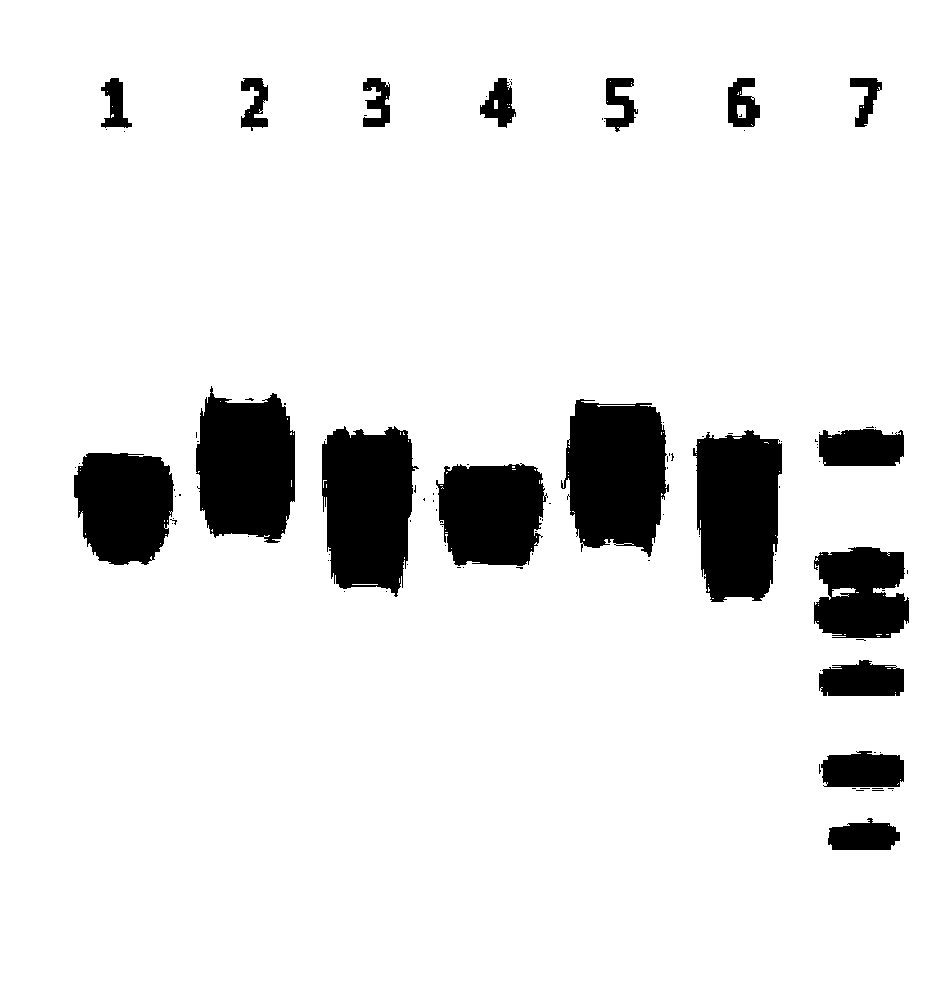Method for constructing spontaneous hyperuricemia mouse model and application of mouse model
A hyperuricemia, mouse model technology, applied in the biological field, can solve problems such as inability to simulate human pathogenesis, gene functional inactivation, early termination of protein reading frames, etc., to achieve long-term experiments and long survival time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the construction of spontaneous hyperuricemia mouse model
[0048] In this example, the urate oxidase gene of C57BL / 6J mice was knocked out by using the technique of transcription activator-like effector nuclease (TALEN), and the homozygous mice obtained by knocking out the urate oxidase gene were spontaneous hyperuricemia Hyperemia mouse model.
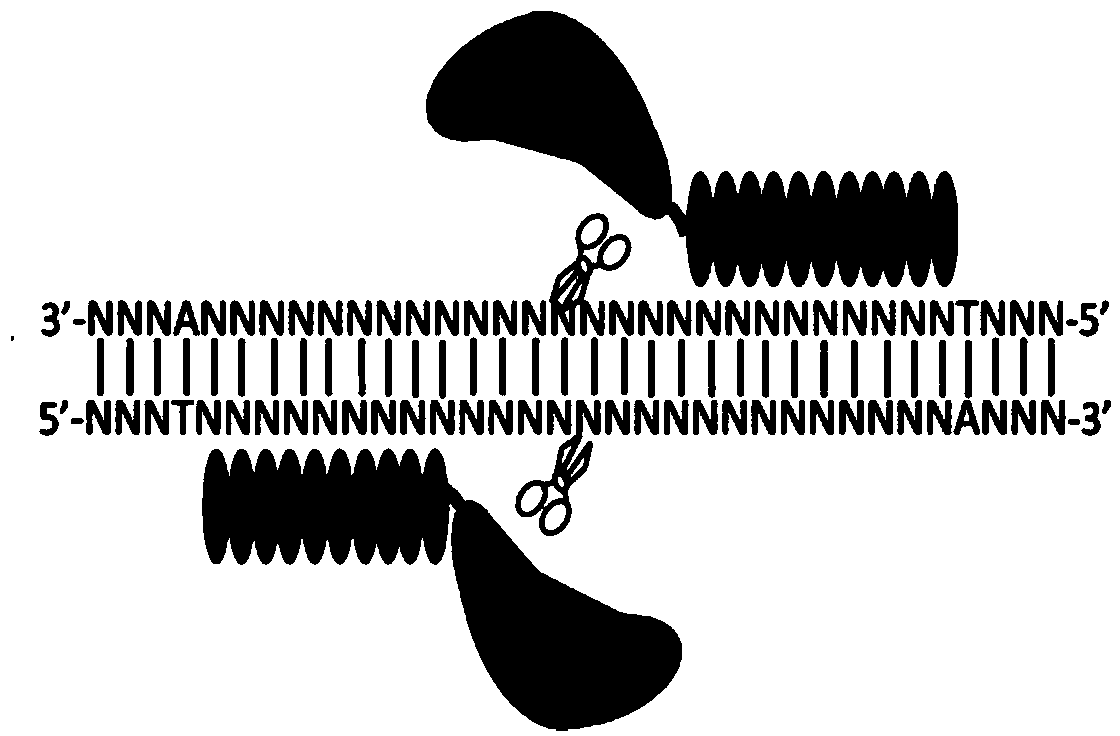
[0049] 1. TALEN site design
[0050] The mouse urate oxidase gene (MGI: 98907; gene chromosome location: Chr3: 146597077-146632305bp, +strand) can produce 4 transcripts, 2 of which can encode proteins. Urate oxidase-001, urate oxidase-003 were 303aa and 199aa, respectively. The inventors of the present invention designed a TALEN for the third exon of urate oxidase-001. The sequence information of this exon is as follows (the two target sequences of the designed TALEN are underlined, and the middle region is the spacer region of the TALEN) :
[0051] 5'-ATCAGAAACATCGAGACCT TTGCAATGAACATCT GTGAGCACTTCCTCTC ...
Embodiment 2
[0085] Embodiment 2, phenotype and physiological characteristic identification of F2 generation homozygous mice
[0086] 1. Observation of mouse phenotype
[0087] The F2 generation homozygous mice, heterozygous mice and littermate wild-type mice obtained in Example 1 were all similar to ordinary C57 mice. The coat color is black, the diet and activities are normal, and there is no hypoplasia or deformity. in Figure 8 It is a photo of the 8-week-old male homozygous mouse of the gene knockout obtained by TALEN technology in Example 1.
[0088] 2. Detection of blood uric acid level
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com