Method utilizing catalytic hydrogenation synthesis of nitrobenzene compounds to prepare aniline compounds
A technology of aniline compounds and nitrobenzenes, which is applied in the field of catalytic hydrogenation to synthesize aniline compounds, can solve problems such as low efficiency and long catalytic reaction time, and achieve the effects of improving reaction efficiency, shortening reaction time, and easy operation control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
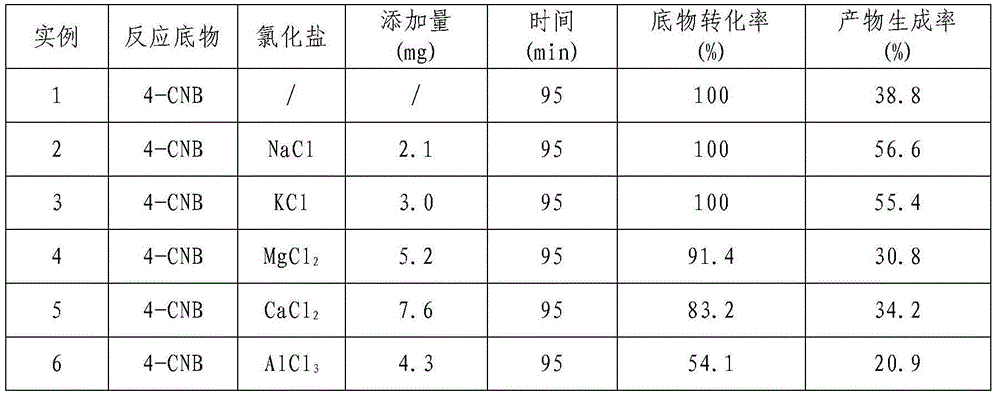
[0022] Example 1 Effect of Different Chloride Salts on the Raney Ni Catalyzed Hydrogenation of p-Chloronitrobenzene (4-CNB) to Synthesize p-Chloroaniline (4-CAN)
[0023] Measure 80mL of 4-chloronitrobenzyl alcohol solution with a concentration of 10g / L and add it to a 100mL three-necked flask, then weigh 0.2g Raney Ni catalyst; add different chloride salts according to 1mol% of the amount of the substrate substance into the reaction system; with N 2 The air in the system was replaced three times, followed by H 2 Replace three times; then pass H 2 , H 2 The flow rate was controlled at 10mL / min, the reaction temperature was controlled at 40°C, and the reaction pressure was normal pressure. The reaction was carried out for 3 hours under continuous stirring with a magnetic stirrer at a speed of 1500r / min. The specific experimental results are shown in Table 1.
[0024] Table 1 Effect of different chlorides on the catalytic hydrogenation of 4-CNB
[0025]
Embodiment 2
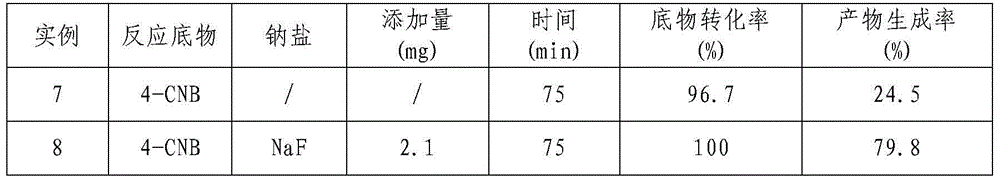
[0026] Example 2 Effects of different sodium salts on Raney Ni catalyzed 4-CNB hydrogenation to synthesize 4-CAN
[0027] Measure the 4-chloronitrobenzyl alcohol solution that 80mL concentration is 10g / L and join in the there-necked flask of 100mL, then take by weighing 0.2g Raney Ni catalyst; In the reaction system; use N 2 The air in the system was replaced three times, followed by H 2 Replace three times; then pass H 2 , H 2 The flow rate was controlled at 10mL / min, the reaction temperature was controlled at 20-60°C, and the reaction pressure was normal pressure. The reaction was carried out for 3 hours under continuous stirring with a magnetic stirrer at a speed of 1500r / min. The specific experimental results are shown in Table 2.
[0028] Table 2 Effects of different sodium salts on the catalytic hydrogenation of 4-CNB
[0029]
[0030]
Embodiment 3
[0031] Example 3 Effect of different NaF additions on Raney Ni catalyzed 4-CNB hydrogenation to synthesize 4-CAN
[0032] Measure 80mL of 4-chloronitrobenzyl alcohol solution with a concentration of 10g / L and add it to a 100mL three-neck flask, then weigh 0.2g Raney Ni catalyst; add different amounts of NaF to the reaction system according to the percentage of the amount of substrate In, add 0mol%, 1mol%, 5mol%, 10mol% NaF respectively; Use N 2 The air in the system was replaced three times, followed by H 2 Replace three times; then pass H 2 , H 2 The flow rate was controlled at 10mL / min, the reaction temperature was controlled at 40°C, and the reaction pressure was normal pressure. The reaction was performed for 3 hours under continuous stirring with a magnetic stirrer at a speed of 1500r / min. The specific experimental results are shown in Table 3.
[0033] Table 3 Effect of different NaF additions on the catalytic hydrogenation of 4-CNB
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com