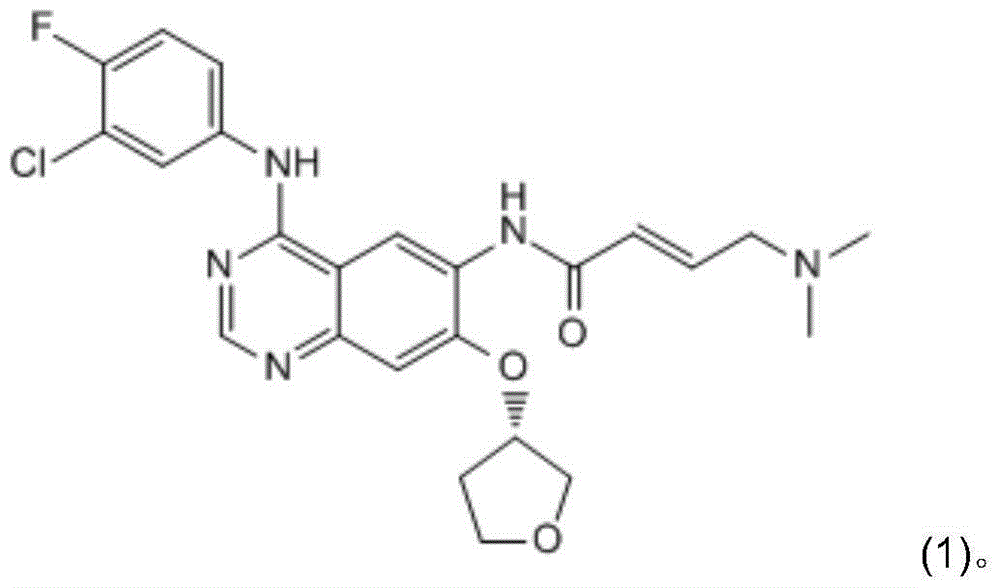
Crystallization purification method
A technology of crystallization and crystallization, applied in the direction of organic chemistry, etc., can solve the problems of difficulty in reducing, low product yield, unfavorable solvent recovery, etc., and achieves the effect of easy operation and high product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0016] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0017] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0018] In the present invention, g means gram, mL means milliliter, min means minute, and mmol means millimole.
Embodiment 1
[0020] Take 10.0g of afatinib crude product and add it to 50mL of isobutyl acetate, stir, and heat to 70°C. After stirring at ℃ for 5 hours, it was filtered, and the obtained solid was washed 3 times with isobutyl acetate, 20 mL each time. The obtained solid was vacuum-dried to dryness at 50° C. to obtain 9.4 g of afatinib with a purity of 99.81%, and a cis-isomer purity of 0.03%.
Embodiment 2
[0022] Take 40.0g of crude afatinib and add it to 400mL of isobutyl acetate, stir, heat to 80°C, dissolve the solution and filter it while it is hot, and cool the obtained filtrate to 25°C-35°C within 3 hours to 4 hours. After stirring at 25°C-35°C for 5 hours, it was filtered, and the obtained solid was washed with isobutyl acetate three times, 80 mL each time. The obtained solid was vacuum-dried to dryness at 60°C to obtain 36.79 g of afatinib with a purity of 99.84% and a cis-isomer of 0.03%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com