Compositions and methods comprising sequences having meganuclease activity
A technology of meganuclease and nucleotide sequence, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0894] Maize Embryo Transformation
[0895] Transformation can be accomplished by various methods known to be effective in plants, including particle-mediated delivery, Agrobacterium-mediated transformation, PEG-mediated delivery, and electroporation.
[0896] a. Particle-mediated delivery
[0897] Transformation of immature maize embryos using particle delivery was performed as follows. The medium recipe follows the following.
[0898] Ears were dehulled and surface sanitized in 30% Clorox bleach plus 0.5% Micro detergent for 20 minutes, then rinsed twice with sterile water. The immature embryos were separated and placed with the hypocotyl side down (cotyledon disc side up), 25 embryos per plate, cultured on 560Y medium for 4 hours, and then aimed at the 2.5cm target area to prepare for bombardment. Alternatively, placed on 560Y at 26°C for 4 hours prior to bombardment as described above, prior to placing isolated embryos on 560L (induction medium) at a temperature fro...
example 2
[0913] Transient expression of BBM-enhanced transformation
[0914] Parameters of the transformation protocol can be modified to ensure that BBM activity is transient. One such method involves precipitating a BBM-containing plasmid in a manner that permits transcription and expression, but precludes subsequent DNA release, for example by chemical PEI.
[0915] In one example, the BBM plasmid was precipitated on gold particles with PEI, and the transgene expression cassette to be integrated (UBI::moPAT-GFPm::PinII; moPAT is a maize-optimized PAT gene) was deposited on gold particles.
[0916]Briefly, gold particles were coated with PEI as follows. First, the gold particles are washed. Weigh 35 mg of gold particles, average diameter (A.S.I. #162-0010) 1.0 in a microcentrifuge tube, add 1.2 ml of absolute ethanol and vortex for 1 minute. The centrifuge tubes were incubated at room temperature for 15 minutes and then centrifuged at 4°C for 15 minutes at high speed using a mi...
example 3
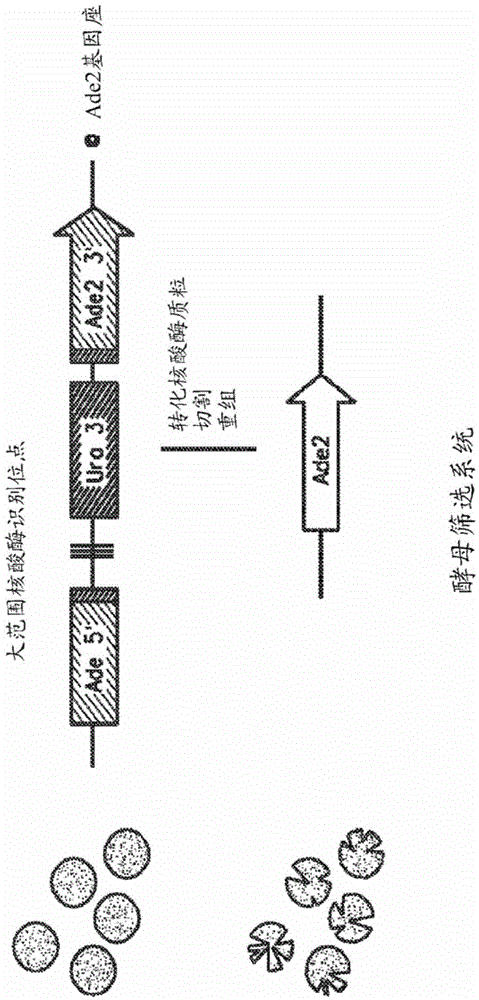
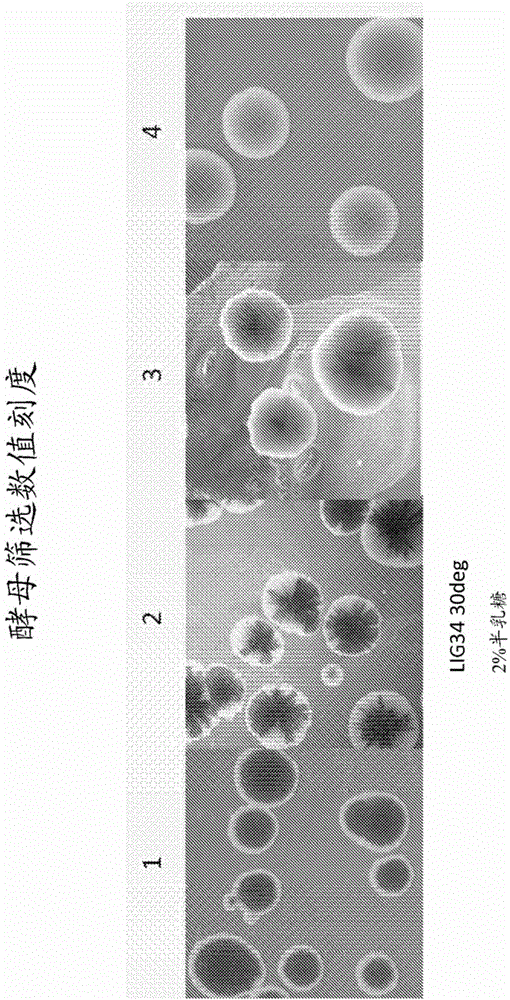
[0921] DNA shuffling to create variants of the LIG3-4 meganuclease
[0922] A. LIG3-4 meganuclease and LIG3-4 recognition sequence
[0923] An endogenous maize genome target site (SEQ ID NO: 2) containing the LIG3-4 recognition sequence was selected for the design of custom double-strand break inducers. The LIG3-4 recognition sequence is a 22bp polynucleotide having the following sequence: ATATACCTCACACGTACGCGTA (SEQ ID NO: 2).
[0924] The wild-type l-Crel meganuclease (SEQ ID NO: 3) was modified as described in US Patent Publication 2009-0133152A1 to produce a LIG3-4 meganuclease designed to recognize the LIG3-4 recognition sequence. The wild-type l-Crel meganuclease is a homodimer. To recognize the LIG3-4 recognition sequence, different substitutions were made for each monomer and for each monomer, the coding sequence was linked with a linker sequence to make a single chain fusion polypeptide (LIG3-4, SEQ ID NO: 1).
[0925] B. Creation of LIG3-4 meganuclease varia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com