Application of LCA in preparation of drug for treating joint inflammation or cartilago articularis and bone destruction
An inflammation and drug technology, applied in the application field of LCA in the preparation of drugs for the treatment of joint inflammation or articular cartilage and bone destruction, to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
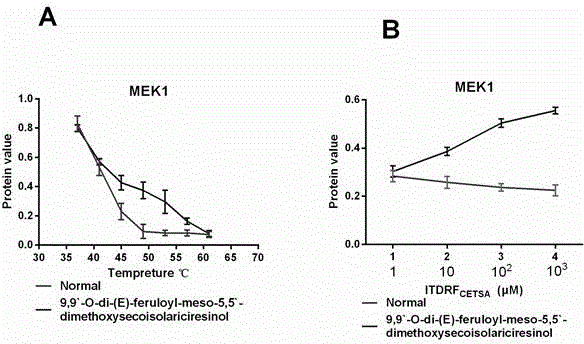
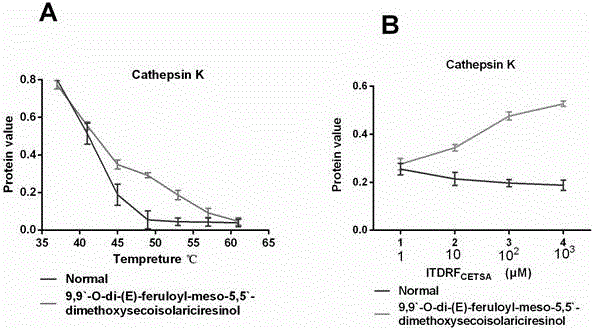
[0020] Example 1 Research on the target of compound LCA
[0021] The target effect of the compound LCA was studied, and the intracellular binding of the compound to MEK1 (RAW264.7 cell) and cathepsin K (Osteoclast) was studied using cellular thermal shift assay (CETSA).
[0022] 1 Experimental method
[0023] 1.1 Osteoclast induction
[0024] Five newborn 3-day-old SD mice were sacrificed, soaked in 75% ethanol solution for 5-10 min, and the tibia of the limbs were removed under the ultra-clean bench, and the bone marrow cells in the bone marrow cavity were absorbed by 1 mL syringe to absorb α-MEM medium. Rinse out, collect the washing liquid, centrifuge at 1200 rpm / min for 10 min, and wash the cells twice with PBS to obtain fresh bone marrow mononuclear cells. The cells were cultured with α-MEM containing 5 ng / mL M-CSF and 10% fetal bovine serum at a cell concentration of 10 7 -10 9 pc / mL, 37℃, 5% CO 2Incubate for 24 h in the incubator. Transfer the unattached cells t...
Embodiment 2
[0033] Example 2 Animal Experiments of Compound LCA in Treating Rheumatoid Arthritis
[0034] 1 Experimental materials
[0035] 1.1 Experimental animals
[0036] Wistar rats, female, 7-8 weeks old, weighing 160-180g, 2 / cage, were reared in separate cages, the rats were free to drink water and eat, the room temperature was 25±1°C, and the animals were adaptively fed for 5 days before the experiment.
[0037] 1.2 Main reagents
[0038] LCA injection: The compound LCA was isolated and obtained according to the prior art. It was formulated into an injection with a concentration of 15 mg / ml in 30% propylene glycol solvent and stored in a 4°C refrigerator for later use.
[0039] MTX injection: specification 5mg / bottle, approval number H20080251, Ebewe Pharma Ges.m.b.H.Nfg.KG.
[0040] Bovine type II collagen was purchased from Chondrex, Inc.; glacial acetic acid was purchased from Beijing Chemical Reagent Company; complete Freund's adjuvant and incomplete Freund's adjuvant were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com