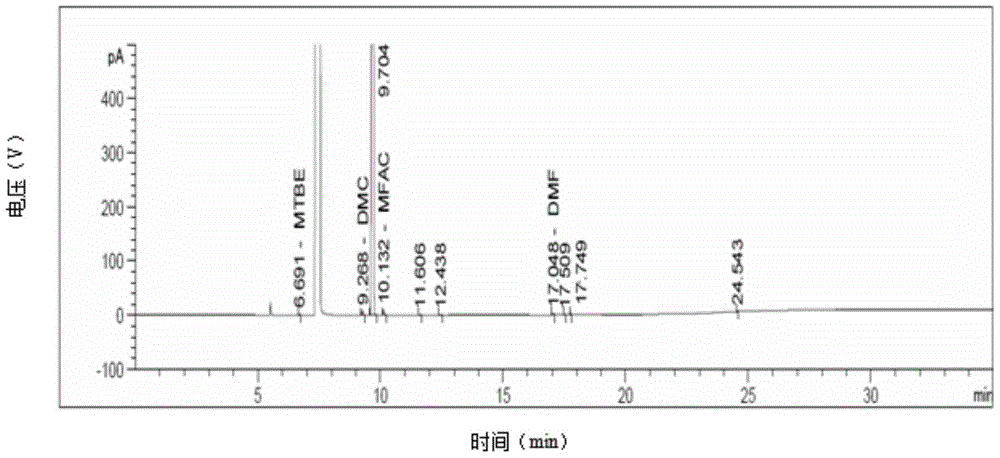
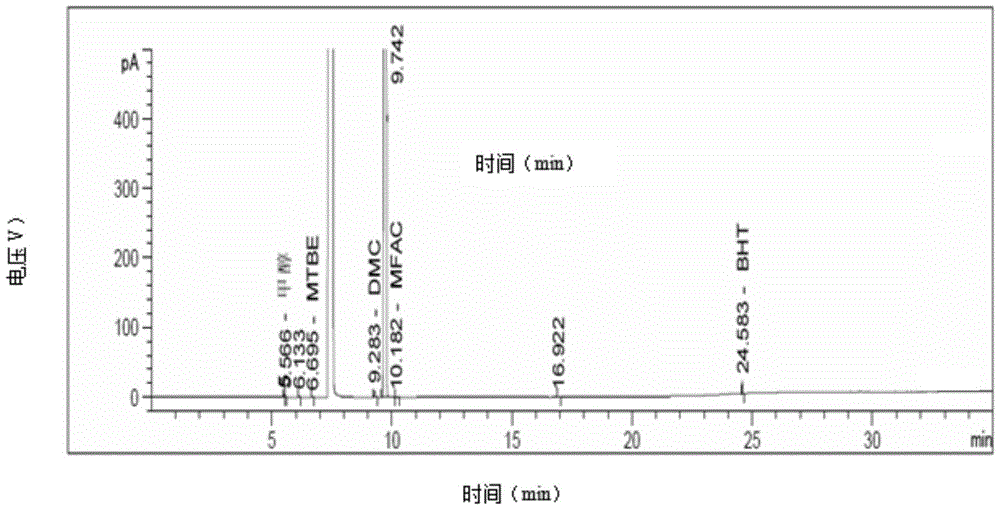
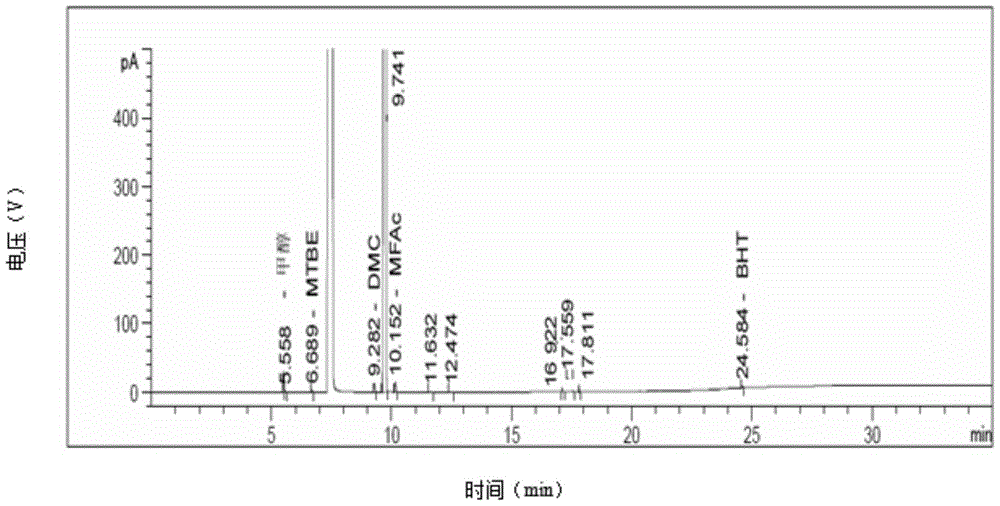
Compound preparation method
A technology for compounds and by-products, applied in the field of preparation of compounds represented by formula 1, can solve the problems of cumbersome process operation, large product loss, and many operation steps, and achieve the effects of simplifying process operation, improving production efficiency, and concise synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] a. Synthesis of methyl 2-fluoroacrylate
[0063] Add 184.2g (2.0mol) of methyl fluoroacetate, 256.0g (2.16mol) of dimethyl oxalate and 1104.0g (14.1mol) of dimethyl sulfoxide into a 3000mL reaction flask, replace with nitrogen twice, and stir at room temperature Dissolve, after the reaction solution is dissolved and clarified, start to add 124.3g (2.3mol) of sodium methoxide solid in batches, control the temperature at 15-25 degrees Celsius, and complete the addition after 1 hour, start to concentrate the reaction solution under reduced pressure, vacuum degree ≥ 0.095Mpa, and react The temperature of the liquid is 35 degrees Celsius. After concentrating for two hours, the sampling control is carried out. The content of methanol is 9.4%, and the content of methyl fluoroacetate is 0.3%. 0.1g of p-cresol, then add 60g (2.0mol) of paraformaldehyde in batches, control the temperature at 20-25 degrees Celsius, after the addition is completed, keep stirring for 1 hour, and con...
Embodiment 2
[0067] a. Synthesis of methyl 2-fluoroacrylate
[0068] Add 184.2 g (2.0 mol) of methyl fluoroacetate, 272.0 g (2.3 mol) of dimethyl oxalate and 736.0 g (9.4 mol) of dimethyl sulfoxide into a 3000 mL reaction flask, replace with nitrogen twice, and stir at room temperature Dissolve, after the reaction solution is dissolved and clarified, start to add 161.3g (2.3mol) of potassium methoxide solid in batches, control the temperature at 15-25 degrees Celsius, and complete the addition after 1 hour, start to concentrate the reaction solution under reduced pressure, vacuum degree ≥ 0.095Mpa, and react The temperature of the liquid is 25 degrees Celsius. After concentrating for two hours, the sampling control is carried out. The content of methanol is 8.0%, and the content of methyl fluoroacetate is 0.1%. 0.1 g of butyl-p-cresol, then add 60 g (2.0 mol) of paraformaldehyde in batches, control the temperature at 20-25 degrees Celsius, after the addition is completed, heat and stir for...
Embodiment 3
[0072] a. Synthesis of methyl 2-fluoroacrylate
[0073] Add 184.2g (2.0mol) of methyl fluoroacetate, 256.0g (2.16mol) of dimethyl oxalate and 1104.0g (14.1mol) of dimethyl sulfoxide into a 3000mL reaction flask, replace with nitrogen twice, and stir at room temperature Dissolve, after the reaction solution is dissolved and clarified, start to add 140.5g (2.6mol) of sodium methoxide solid in batches, control the temperature at 5-15 degrees Celsius, and complete the addition after 1 hour, start to concentrate the reaction solution under reduced pressure, vacuum degree ≥ 0.095Mpa, and react The temperature of the liquid is 28 degrees Celsius. After concentrating for two hours, the sampling control is carried out. The content of methanol is 9.0%, and the content of methyl fluoroacetate is 0.05%. 0.1 g of p-cresol, then add 60 g (2.0 mol) of paraformaldehyde in batches, and control the temperature at 10-15 degrees Celsius. -The content of methyl fluoroacrylate is 10.5%, and the yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com