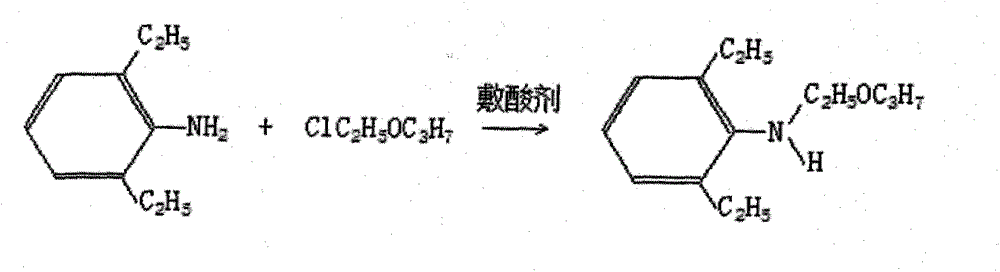
Method for producing 2, 6-diethyl-N-(2-propoxyethyl)phenylamine serving as pretilachlor intermediate
A technology of propoxyethyl and diethylaniline, applied in the field of herbicides, can solve the problems of high manufacturing cost and low rectification efficiency, and achieve the effects of reducing production cost, good industrial value, and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] The technical solutions in the embodiments of the present invention will be described clearly and completely below. Obviously, the described embodiments are only a part of the embodiments of the present invention, rather than all the embodiments. Based on the embodiments of the present invention, all other embodiments obtained by those of ordinary skill in the art without creative work shall fall within the protection scope of the present invention.
[0011] The embodiment of the present invention discloses a method for producing 2,6-diethyl-N-(2-propoxyethyl)aniline, an intermediate of pretilachlor, which not only has the characteristics of short process flow, but also can improve the utilization of raw materials Rate and reduce production costs, including:
[0012] 2,6-Diethyl aniline, chloroethyl propyl ether and acid binding agent react under normal pressure or under pressure to form 2,6-diethyl-N-(2-propoxyethyl)aniline; The molar ratio of 2,6-diethylaniline, chloroeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com