Method for producing 1,4-benzoxazine compound
A technology of benzoxazine and a manufacturing method, which is applied in the field of 1,4-benzoxazine compounds and can solve problems such as difficulty in reasonably predicting the existence of crystal polymorphisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] (1) N-(2,2-Dimethyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-7-yl)-N-(methylsulfonyl) Manufacture of methanesulfonamide
[0161] [chemical formula 24]
[0162]
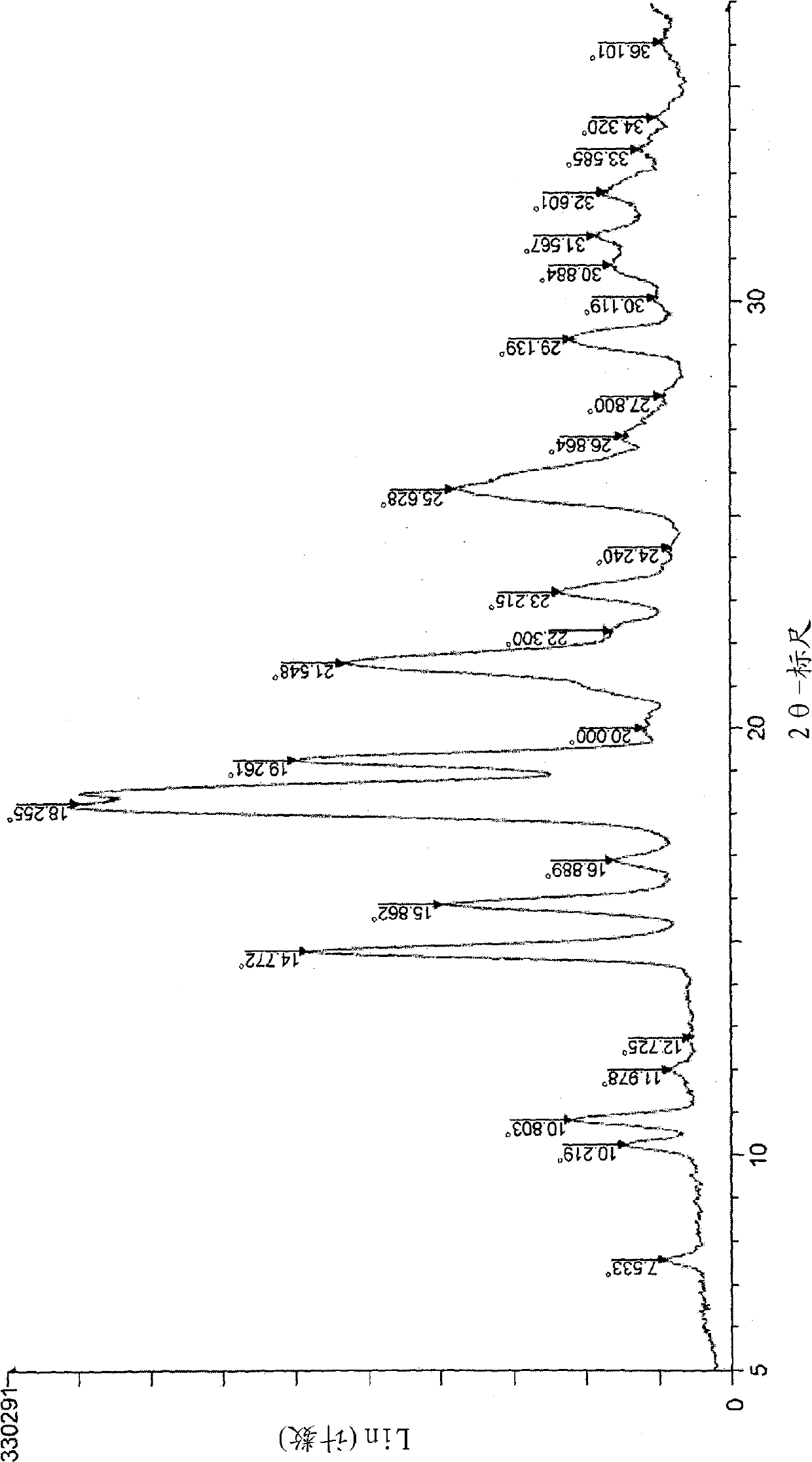
[0163] To a suspension of 7-amino-2,2-dimethyl-2H-1,4-benzoxazin-3-(4H)-one (150 g) in acetonitrile (1500 mL) was added dropwise triethylamine at 40 °C (79 g), and methanesulfonyl chloride (89.4 g) was added dropwise to the mixture (internal temperature: 39 to 50° C.), followed by stirring at this temperature for 20 minutes. After adding triethylamine (79 g) and methanesulfonyl chloride (89.4 g) dropwise to the reaction mixture in this order (inner temperature 42-50 degreeC), it stirred at this temperature for 25 minutes. Triethylamine (39.5 g) and methanesulfonyl chloride (44.7 g) were further added dropwise to the reaction mixture in this order (internal temperature: 42 to 47° C.), followed by stirring at 40° C. for 4 hours. Water (1500 mL) was added dropwise to the reaction mixture, and after stirring the m...
Embodiment 2
[0176] N-[4-(4-fluorophenyl)-2,2-dimethyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-7-yl]methanesulfonate Production of amides
[0177] [chemical formula 27]
[0178]
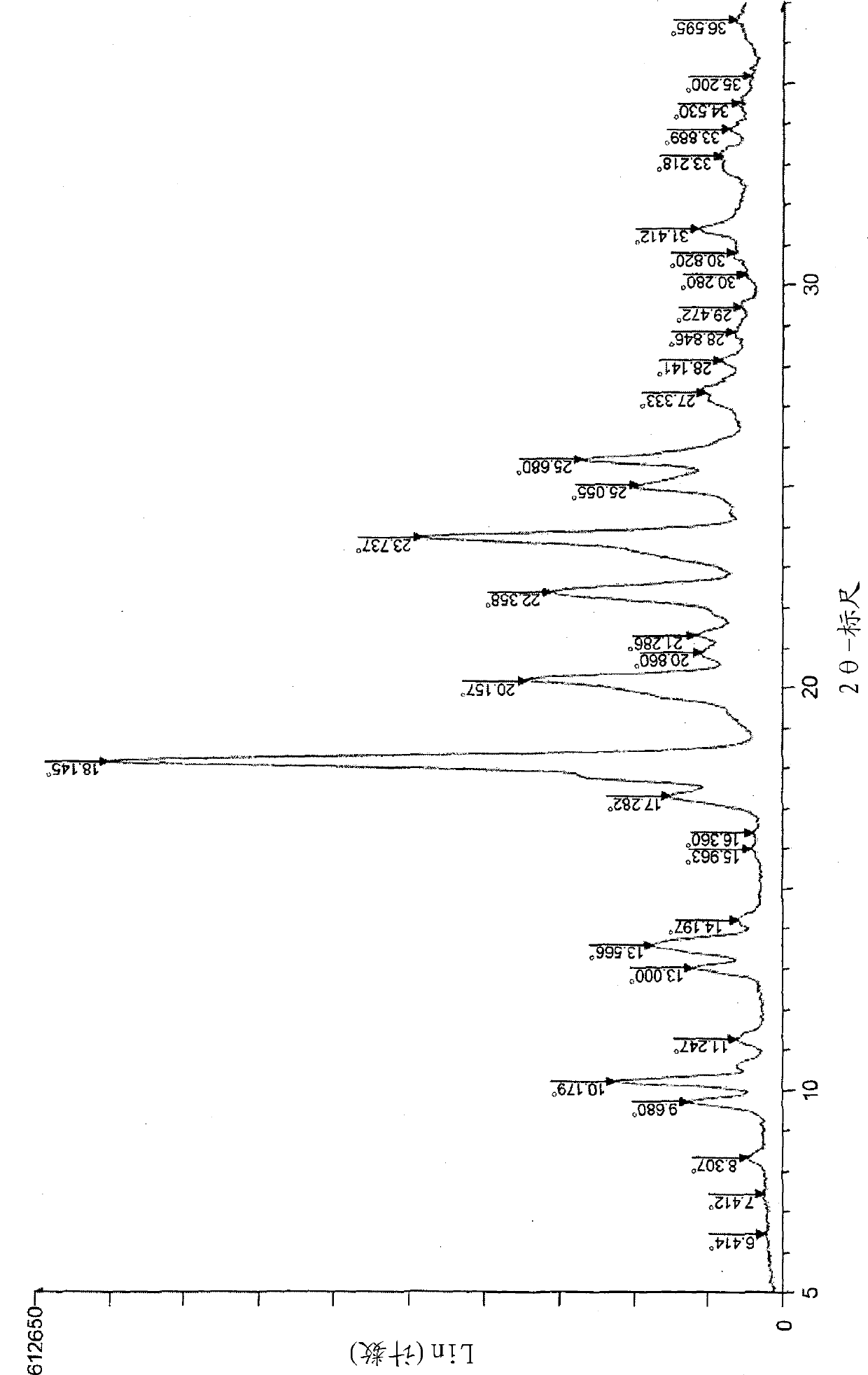
[0179] To N-[4-(4-fluorophenyl)-2,2-dimethyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-7-yl]- A suspension of N-(methylsulfonyl)methanesulfonamide (1 g) in acetone (3 mL) was added dropwise with aqueous sodium hydroxide solution (0.18 g / 3 mL) at 25°C, and the mixture was stirred at that temperature for 90 minutes. Concentrated hydrochloric acid / acetone / water (0.24 g / 0.5 mL / 0.35 mL) was added dropwise to the reaction mixture at 25° C., and the mixture was stirred for 2 hours. The precipitated crystals were collected by filtration, washed with acetone / water (1:1.6mL) solution, and dried under reduced pressure at 50°C to obtain the title compound (0.79g) as Form A crystals (yield: 95%, purity: 100 %).
[0180] M.p.: 240°C.
Embodiment 3
[0182] N-[4-(4-fluorophenyl)-2,2-dimethyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-7-yl]methanesulfonate Production of amides
[0183] [chemical formula 28]
[0184]
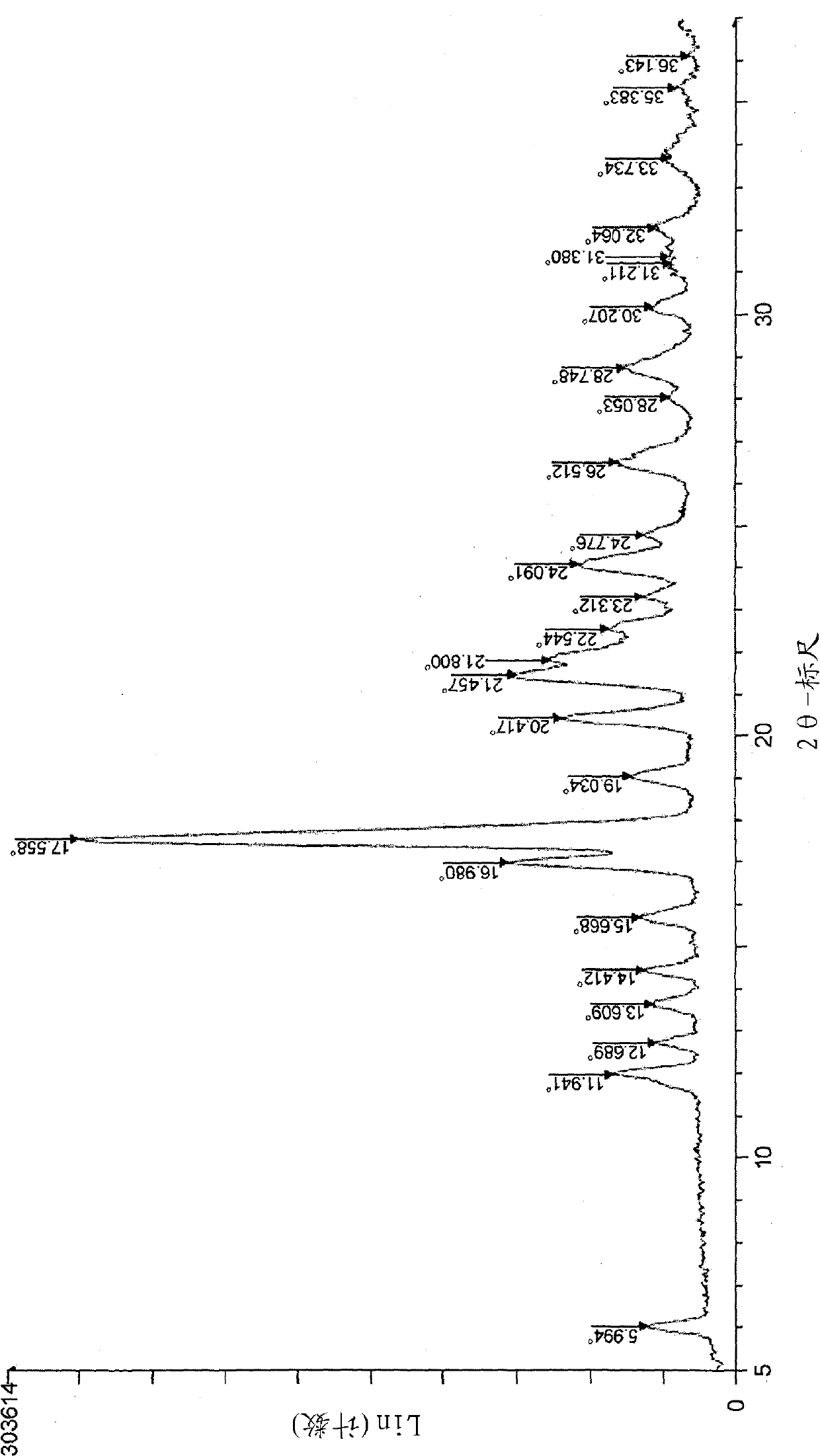
[0185] To N-[4-(4-fluorophenyl)-2,2-dimethyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-7-yl]- After the suspension of N-(methylsulfonyl)methanesulfonamide (1g) in dimethylsulfoxide (3mL) was added dropwise at 25°C in aqueous sodium hydroxide solution (0.18g / 3mL), dimethylsulfoxide / water (1:1.2 mL), and the mixture was stirred overnight at this temperature. Concentrated hydrochloric acid / dimethylsulfoxide / water (0.24g / 0.5mL / 0.35mL) was added dropwise to the reaction mixture at 25°C, the mixture was stirred for 5 hours, the precipitated crystals were collected by filtration, and dimethylsulfoxide / After washing with water (1:1.6 mL) solution and 10 mL of water, and drying under reduced pressure at 50°C, the title compound (0.81 g) was obtained as Form A crystal (yield: 98%, purity: 100%)
[0186] M.p.: 240°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com