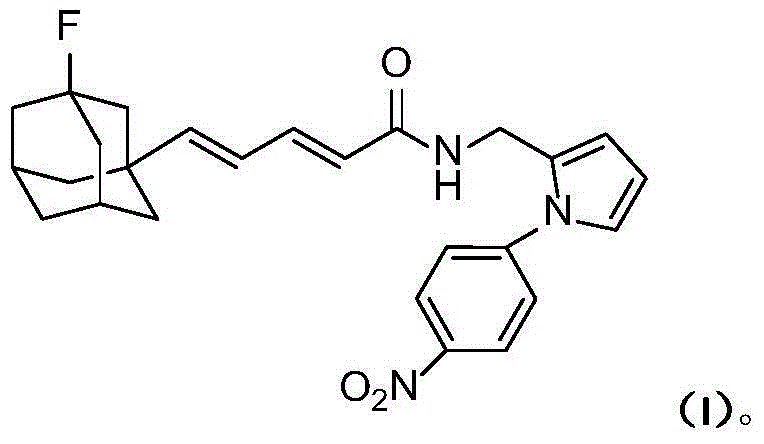
Compound containing nitrobenzene and diene fluoro adamantane structure and preparation method thereof
A compound and diene technology, applied in the field of drugs related to thrombotic diseases, can solve problems such as high bleeding risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
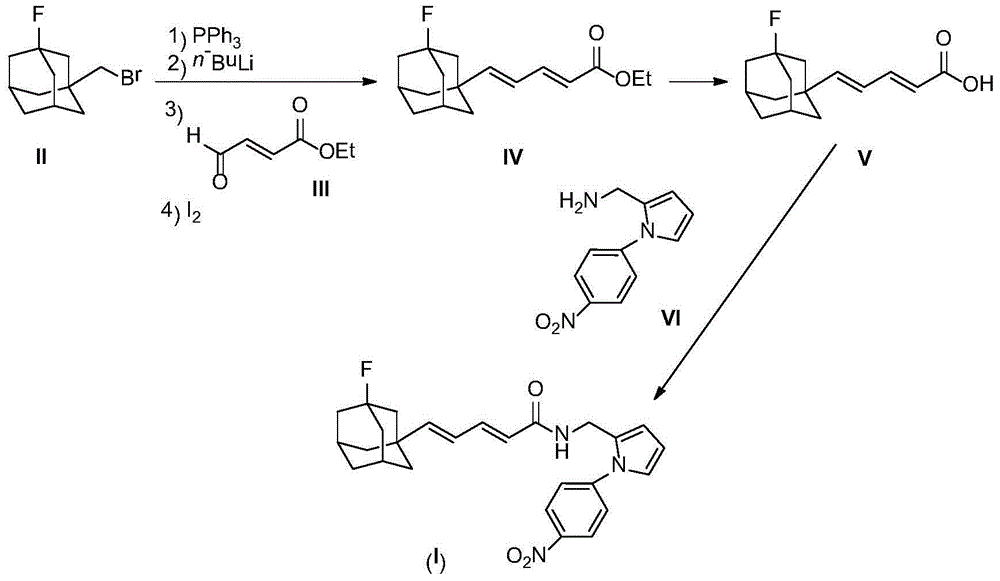
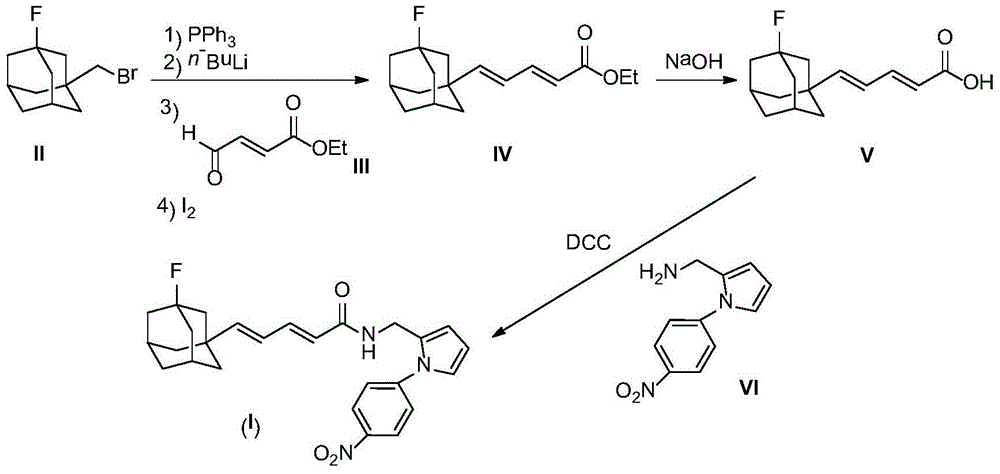
[0022] 2.46g (10mmol) compound II and 2.62g (10mmol) PPh 3 Dissolve in 20 mL of dry THF and reflux overnight under nitrogen protection. After the reaction mixture was cooled to room temperature, a white cloudy solution was obtained. Cooled to -78°C under nitrogen protection, slowly added 6.25mL (10mmol, 1.6M) n-BuLi n-hexane solution dropwise. After the dropwise addition was completed, stirring was continued for one hour, and a solution prepared by dissolving compound 1.28g (10mmol) III in 2mL of THF was added dropwise. After the dropwise addition was complete, the reaction mixture was slowly warmed to room temperature and then refluxed for 1 hour. The reaction mixture was poured into ice water, stirred, extracted with 50 mL×3 dichloromethane, the combined extracts were washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, 0.50 g of iodine was added to the filtrate, and stirred overnight at room temperature....
Embodiment 2
[0025] Example 2 Preparation of Reference Compound D1
[0026] In order to fully illustrate the beneficial effects of the compounds of the present invention, the applicant recorded the following compound D1 (unpublished) found during the experiment as a pharmacodynamic reference compound.
[0027]
[0028] The synthesis method is as follows:
[0029]
[0030] 2.47g (10mmol) compound II and 2.62g (10mmol) PPh 3 Dissolve in 20 mL of dry THF and reflux overnight under nitrogen protection. After the reaction mixture was cooled to room temperature, a white cloudy solution was obtained. Cooled to -78°C under nitrogen protection, slowly added 6.25mL (10mmol, 1.6M) n-BuLi n-hexane solution dropwise. After the dropwise addition was completed, stirring was continued for one hour, and a solution prepared by dissolving compound 1.28g (10mmol) III in 2mL of THF was added dropwise. After the dropwise addition was complete, the reaction mixture was slowly warmed to room temperature...
Embodiment 3
[0033] Example 3 In vitro platelet aggregation inhibition test
[0034]Pharmacological tests of substances were performed in TRAP (thrombin receptor activating peptide)-induced platelet aggregation in 96-well plates. 3.13% sodium citrate solution was added to the syringe in advance, and then 20 mL of blood from healthy volunteers was drawn in, centrifuged at 1500 g for 20 minutes, platelet-rich plasma (PRP) was separated and mixed with 1 μL of PGE1 solution (500 μg / mL ethanol solution) / mL PRP for treatment. After incubation at room temperature for 5 minutes, they were centrifuged at 1200 g for 20 minutes to remove leukocytes. Transfer the leukocyte-free PRP to 15 mL PP tubes in batches at 5 mL / portion, and centrifuge at 3600 g to pellet the platelets. Then, decant the upper plasma layer and resuspend the platelet pellet from 5 mL of PRP in 1 mL of Tyrode (120 mM NaCl, 2.6 mM KCl, 12 mM NaHCO 3 , 0.39mM NaH 2 PO 4 , 10 mM HEPES, 0.35% BSA, 5.5 mM glucose, pH=7.4), and adju...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com