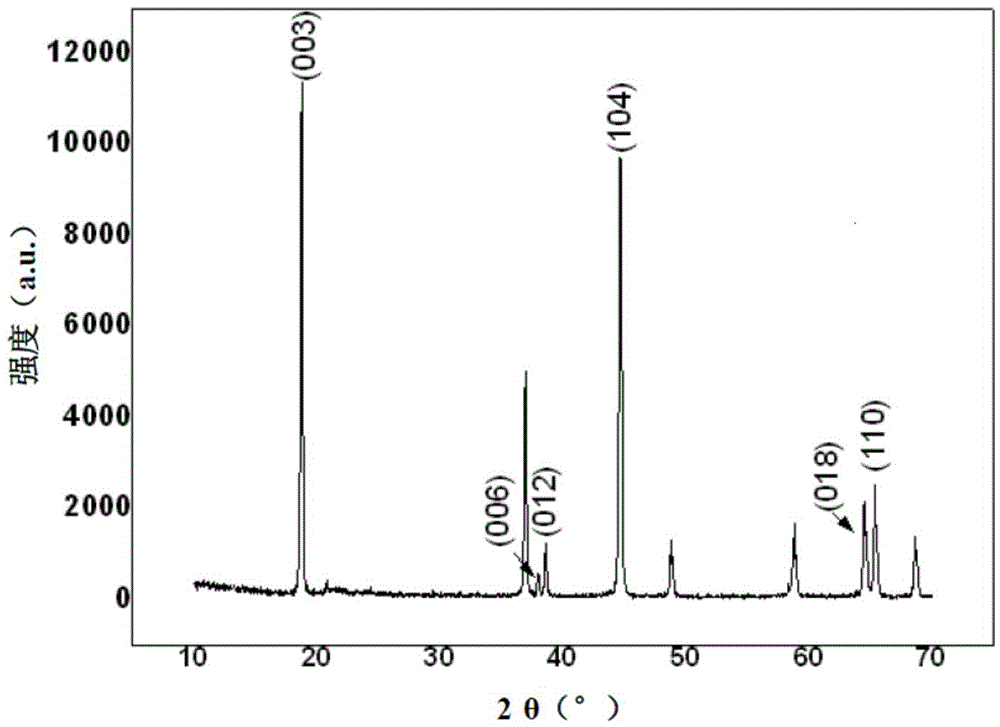
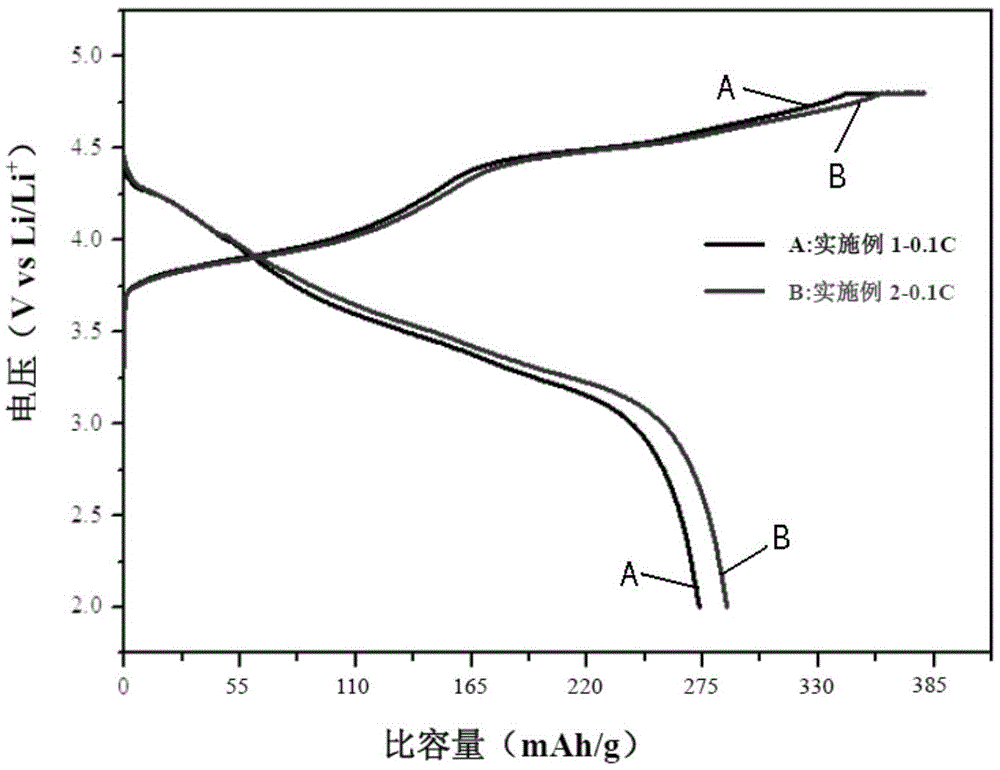
Surface-doped rich lithium cathode material and preparation method thereof
A technology of lithium-rich positive electrode material and surface doping, which is applied in the field of surface-doped lithium-rich positive electrode material and its preparation, can solve the problems of unfavorable rate discharge characteristics of electrode materials, no electrochemical activity of clad materials, etc., and achieve improved structure Stability, improve cycle performance, reduce the effect of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] to xLi 2 MnO 3 ·(1-x)Li[Ni 0.5 mn 0.5 ]O 2 as the matrix, the surface is doped with Rh 3+ , doped with Rh 3+ The surface layer available xLi 2 MnO 3 ·(1-x)LiNi n mn m Rh 1-m-n o 2 Indicates that, where x=0.5, n=0.4, m=0.3, the molar ratio of lithium ions in solution A is measured twice as follows: 1:0.5, the specific operation steps are as follows:
[0023] (1) Configuration solution: Dissolve lithium nitrate and citric acid with a stoichiometric ratio of 1:1 in deionized water to make solution A, in which the lithium ion concentration is 0.50mol / L; 0.25mol nickel nitrate and 0.75mol manganese nitrate Dissolve in deionized water to make solution B; dissolve 0.10mol nickel nitrate, 0.325mol manganese nitrate and 0.075mol rhodium nitrate in deionized water to make solution C.
[0024] (2) Measure 3.00L of solution A, add solution B to 3.00L of solution A, stir at 60°C to form a gel, pre-calcine at 300°C, keep warm for 9 hours, cool naturally and grind to obtai...
Embodiment 2
[0027] to xLi 2 MnO 3 ·(1-x)Li[Ni 0.5 mn 0.5 ]O 2 as the matrix, the surface is doped with Rh 3+ , doped with Rh 3+ The surface layer available xLi 2 MnO 3 ·(1-x)LiNi n mn m Rh 1-m-n o 2 Said, where x=0.5, n=0.4, m=0.3, the following two times measure the molar ratio of lithium ions in solution A to be 1:1, the specific operation steps are as follows:
[0028] (1) Configuration solution: Dissolve lithium nitrate and citric acid with a stoichiometric ratio of 1:1 in deionized water to make solution A, in which the lithium ion concentration is 0.50mol / L; 0.25mol nickel nitrate and 0.75mol manganese nitrate Dissolve in deionized water to make solution B; dissolve 0.20mol nickel nitrate, 0.65mol manganese nitrate and 0.15mol rhodium nitrate in deionized water to make solution C.
[0029] (2) Measure 3.00L of solution A, add solution B to 3.00L of solution A, stir at 60°C to form a gel, pre-calcine at 300°C, keep warm for 9 hours, and then grind to obtain D.
[0030] (...
Embodiment 3
[0032] to xLi 2 MnO 3 ·(1-x)Li[Ni 0.5 mn 0.5 ]O 2 as the matrix, the surface is doped with Rh 3+ , doped with Rh 3+ The surface layer available xLi 2 MnO 3 ·(1-x)LiNin mn m Rh 1-m-n o 2 Said, where x=0.5, n=0.4, m=0.3, the following two times measure the molar ratio of lithium ions in solution A to be 1:1, the specific operation steps are as follows:
[0033] (1) Configuration solution: Dissolve lithium salt and citric acid with a stoichiometric ratio of 1:1 in deionized water to form solution A, in which the lithium salt is a mixture of lithium nitrate and lithium hydroxide with a stoichiometric ratio of 1:1, and lithium The ion concentration is 0.50mol / L; 0.25mol nickel nitrate and 0.75mol manganese nitrate are dissolved in deionized water to make solution B; 0.20mol nickel nitrate, 0.65mol manganese nitrate and 0.15mol rhodium nitrate are dissolved in deionized water to prepare into solution C.
[0034] (2) Measure 3.00L of solution A, add solution B to 3.00L of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com