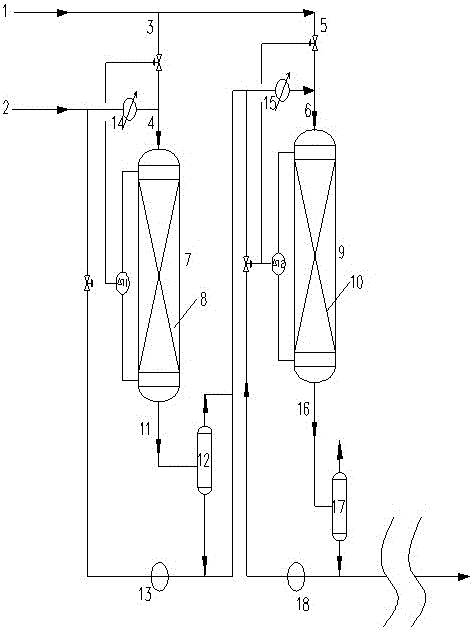
A kind of hydrogenation process of producing hydrogen peroxide by anthraquinone method
A technology of hydrogen peroxide and anthraquinone method, applied in the direction of peroxide/peroxy hydrate/peroxyacid/superoxide/ozonide, inorganic chemistry, chemical instruments and methods, etc., can solve the problem that the bed temperature cannot be adjusted in time liter, failure to achieve strict or precise control of the hydrogenation reaction process, etc., to achieve the effect of preventing hydrogen shortage, facilitating hydrogenation, and reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Two hydrogenation reactors, each hydrogenation reactor filled with a section of catalyst 0.15m 3. The reaction conditions of the first hydrogenation reactor are as follows: the inlet temperature is 40°C, the top pressure is 0.28-0.34MPa, and the fresh working fluid flow rate is 7.08m 3 / h, the circulating working fluid flow rate is 8.7m 3 / h, the amount of hydrogen is 39.9 Nm 3 / h. The reaction conditions of the second hydrogenation reactor are as follows: the inlet temperature is 46.7°C, the top pressure is 0.23-0.28MPa, and the flow rate of the circulating working fluid is 9.1m 3 / h, the amount of hydrogen is 46.8Nm 3 / h.
[0033] After being treated by this method, the temperature rise of the first hydrogenation reactor is 4.6-4.7°C, the temperature rise of the second hydrogenation reactor is 4.8°C-4.9°C, and the hydrogen efficiency at the outlet of the second hydrogenation reactor is 7.8-8.0g / L. After oxidizing the hydrogen anthraquinone at the outlet of the s...
Embodiment 2
[0035] Two hydrogenation reactors, each hydrogenation reactor filled with a section of catalyst 0.15m 3 . The reaction conditions of the first hydrogenation reactor are as follows: the inlet temperature is 55°C, the top pressure is 0.25-0.30MPa, and the fresh working fluid flow rate is 7.08m 3 / h, the circulating working fluid flow rate is 9.2m 3 / h, the amount of hydrogen is 41.2 Nm 3 / h. The reaction conditions of the second hydrogenation reactor are as follows: the inlet temperature is 62.3°C, the top pressure is 0.23-0.25MPa, and the flow rate of the circulating working fluid is 10.5m 3 / h, the amount of hydrogen is 45.3Nm 3 / h.
[0036] After treatment by this method, the temperature rise of the first hydrogenation reactor is 5.0-5.2°C, the temperature rise of the second hydrogenation reactor is 5.3°C-5.5°C, and the hydrogen efficiency at the outlet of the second hydrogenation reactor is 8.0-8.2g / L. After oxidizing the hydrogen anthraquinone at the outlet of the sec...
Embodiment 3
[0038] Three hydrogenation reactors, each hydrogenation reactor filled with a catalyst 0.1m 3 , the reaction conditions of the first hydrogenation reactor are as follows: the inlet temperature is 40°C, the top pressure is 0.30-0.34MPa, and the fresh working fluid flow rate is 4.78m 3 / h, the circulating working fluid flow rate is 2.68m 3 / h, the amount of hydrogen is 26.6 Nm 3 / h. The reaction conditions of the second hydrogenation reactor are as follows: the inlet temperature is 44.1°C, the top pressure is 0.25-0.28MPa, and the flow rate of the circulating working fluid is 2.79m 3 / h, the amount of hydrogen is 29.5Nm 3 / h. The reaction conditions of the third hydrogenation reactor are as follows: the inlet temperature is 47.5°C, the top pressure is 0.2-0.23MPa, and the flow rate of the circulating working fluid is 2.92m 3 / h, the amount of hydrogen is 30.6Nm 3 / h.
[0039] After being treated by this method, the temperature rise of each hydrogenation reactor was 3.3-3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| crushing resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com