Method and kit for detecting circulating tumor cell antigens in peripheral blood through electrochemical luminescence detection
A technology of peripheral blood circulation and tumor cells, which is applied in the field of electrochemiluminescence detection of peripheral blood circulating tumor cell antigens and kits, can solve the problems that ordinary patients cannot afford routine detection, the detection price is high, and the dynamic monitoring cannot be performed, so as to achieve harmful effects. The effect of small size, improved sensitivity, and improved detection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of EpCAM antibody-labeled magnetic beads
[0027] Mix the streptavidin magnetic beads thoroughly with a Vortex stirrer, then use a microtube to take 100 μL of a uniform particle solution (equivalent to 1 mg of particles); add 2 μg of biotin-labeled EpCAM antibody (antibody can be derived from human, mouse , rabbits, goats and other currently known biological sources or synthetic antibodies, this example uses a solution of mouse anti) and mixes; incubate on a rotating shaker at room temperature for 30 minutes; place the microtube on the magnetic base for about 1 minute, and Remove the supernatant; add 100 μL of washing buffer, wash twice, then place the microtube on the magnetic base for about 1 min, and remove the supernatant; add preservation buffer, mix well, and store at 4°C. The specification of the magnetic beads is that the particle diameter is between 0.5-5um.
Embodiment 2
[0028] Example 2 Preparation of magnetic beads labeled with anti-EpCAM antibody (secondary antibody)
[0029] Mix the streptavidin magnetic beads thoroughly with a Vortex stirrer, then use a microtube to take 100 μL of a uniform particle solution (equivalent to 1 mg of particles); add a solution containing 2 μg of biotin-labeled secondary antibody, and mix well; Incubate on a rotary shaker for 30 min; place the microtube on the magnetic base for about 1 min, and remove the supernatant; add 100 μL of washing buffer and wash 3 times; then add EpCAM antibody solution and mix well; rotate and shake at room temperature Incubate on the bed for 30min; place the microtube on the magnetic base for about 1min, and remove the supernatant; add 100μL of washing buffer, wash twice; then place the microtube on the magnetic base for about 1min, and remove the supernatant ; Add storage buffer, mix well, and store at 4°C.
Embodiment 3
[0030] Example 3 Evaluation of antibody-labeled magnetic beads for CTC enrichment ability one
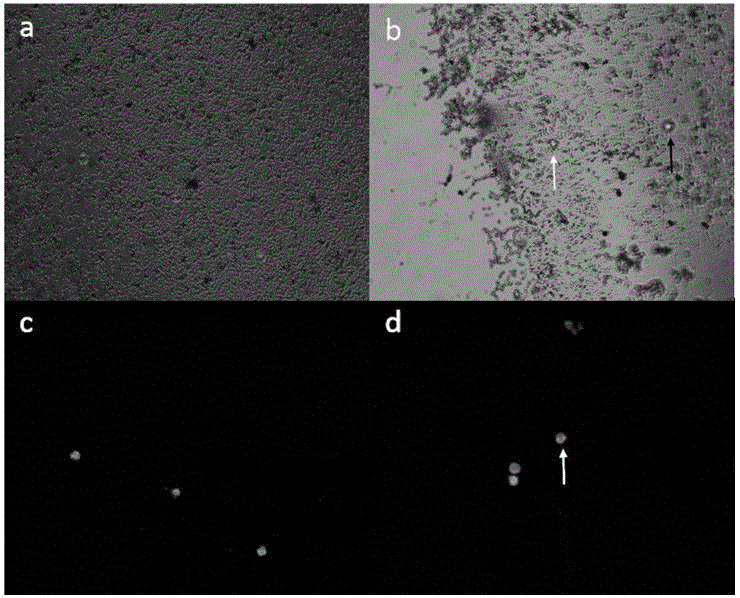
[0031] Put 101 BT474 Her2-positive breast cancer cells into the total system of 200uL, add 2uL EpCAM antibody magnetic beads (you can choose the immunomagnetic beads in Example 1 and Example 2, and use the immunomagnetic beads in Example 1 here , the same below), mix well, and incubate the microtube evenly by inverting for 20min at room temperature; place the microtube on the magnetic base for about 1min, and remove the supernatant; add 200uL of washing buffer, wash 3 times, the microscope counted 61, and the enrichment efficiency was 60.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com