Fullerene-perylene functional molecule and preparation method thereof
A technology of perylene and functional molecules, which is applied in the field of fullerene-perylene functional molecules and their preparation, can solve problems affecting the application of molecules, and achieve the effect of fast reaction and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
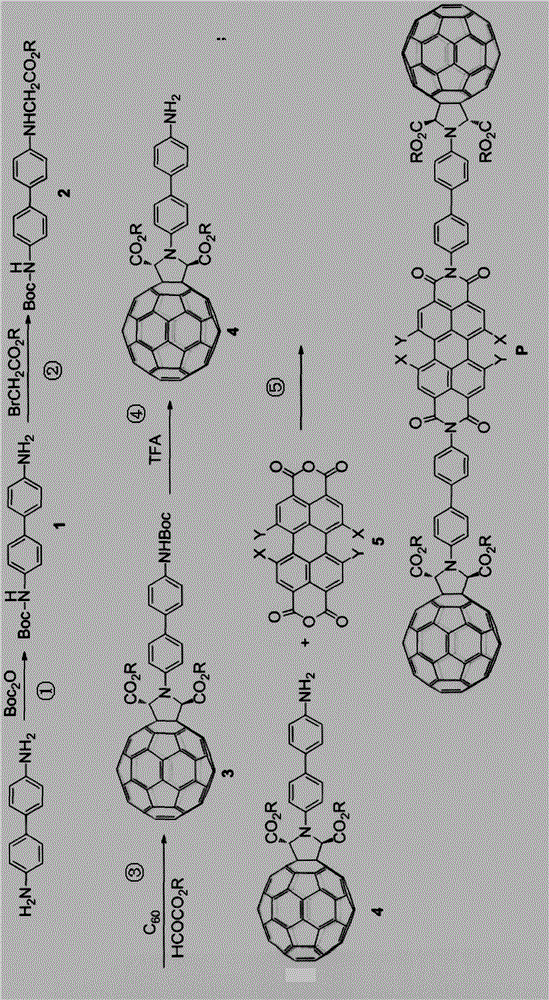
[0051] Example 1 (fullerene-perylene functional molecule P2):
[0052] In the general formula, R is methyl, X is H, Y is -NR 3 H, where R 3 For isobutyl.
example 2
[0053] Example 2 (fullerene-perylene functional molecule P3):
[0054] In the general formula, R is isopropyl, X is Br, Y is SR 3 , where R 3 is a substituted aryl.
example 3
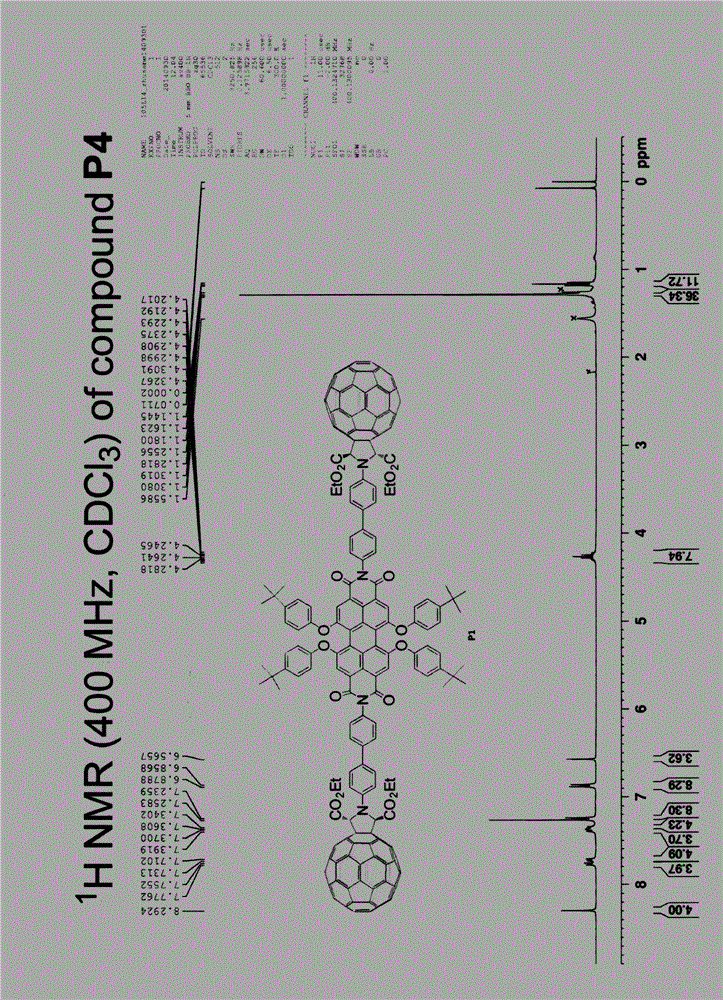
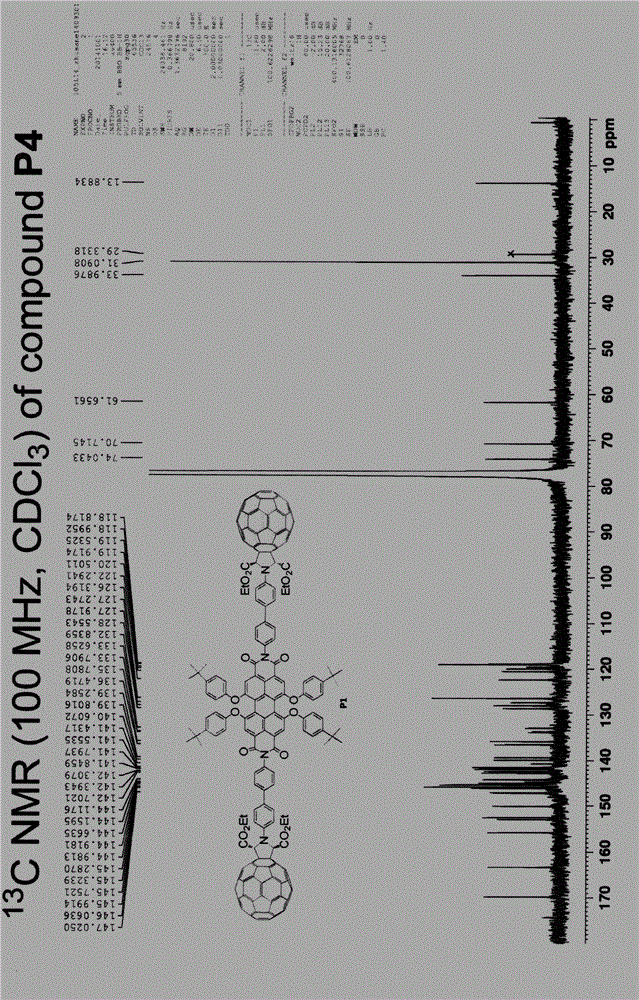
[0055] Example 3 (fullerene-perylene functional molecule P4):
[0056] In the general formula, R is dodecyl, Y is Cl, X is -NR 1 R 2 , where R 1 is a substituted heterocyclic aryl group, R 2 for H.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com