Internal olefin sulfonate composition and detergent composition containing same
A technology of internal olefin sulfonate and composition, applied in detergent compositions, surface active detergent compositions, high-foaming compositions, etc., can solve problems such as insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
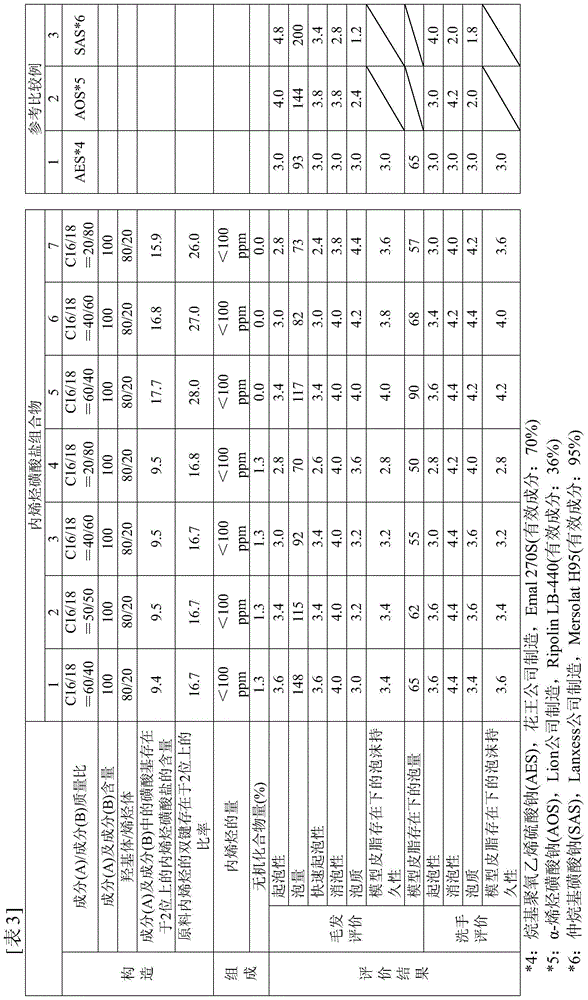
[0092] Hereinafter, the present invention will be specifically described based on examples. In addition, unless otherwise indicated in the table|surface, content of each component shows mass %. In addition, the measurement methods of various physical properties are as follows.
[0093] (1) Measurement conditions
[0094] (i) Determination method of double bond position of internal olefin
[0095] The double bond positions of internal olefins are determined by gas chromatography (hereinafter abbreviated as GC). Specifically, after producing a disulfide derivative by reacting dimethyl disulfide with an internal olefin, each component was separated by GC. The position of the double bond of the internal olefin was determined from the area of each peak.
[0096] In addition, the apparatus and analysis conditions used for the measurement are as follows. GC device (trade name: HP6890, manufactured by HEWLETT PACKARD Co.), column (trade name: Ultra-Alloy-1HT capillary column 30...
manufacture example A
[0112] [Production Example A] Synthesis of internal olefin with 16 carbon atoms and 16.5% by mass of 2-position double bond
[0113] Add 7000 g (28.9 moles) of 1-hexadecanol (product name: Kalcol 6098, manufactured by Kao Corporation) and 700 g of solid acid catalyst γ-alumina (STREM Chemicals, Inc.) (relative to Raw material alcohol (10% by mass) was reacted at 280° C. for 5 hours while blowing nitrogen gas (7000 mL / min.) into the system under stirring conditions. After the reaction, the conversion rate of alcohol was 100%, and the purity of C16 internal olefins was 99.7%. The obtained crude internal olefins were transferred to a distillation flask and distilled at 136-160° C. / 4.0 mmHg to obtain internal olefins having 16 carbon atoms with an olefin purity of 100%. The double bond distribution of the obtained internal olefin was: 0.5% by mass at the C1 position, 16.5% by mass at the C2 position, 15.4% by mass at the C3 position, 16.4% by mass at the C4 position, 17.2% by mas...
manufacture example B
[0114] [Production Example B] Synthesis of internal olefin with 18 carbon atoms and 16.9% by mass of 2-position double bond
[0115] Add 1-octadecyl alcohol (product name: Kalcol 8098, manufactured by Kao Corporation) 7000g (25.9 moles) and solid acid catalyst γ-alumina (STREM Chemicals, Inc) 1050g (relatively The raw material alcohol was 15% by mass), and reacted for 13 hours at 285° C. while flowing nitrogen gas (7000 mL / min.) into the system while stirring. After the reaction, the conversion rate of alcohol was 100%, and the purity of C18 internal olefins was 98.5%. The obtained crude internal olefin was transferred to a distillation flask and distilled at 148-158° C. / 0.5 mmHg to obtain an internal olefin having 18 carbon atoms with an olefin purity of 100%. The double bond distribution of the obtained internal olefin was 0.7% by mass at the C1 position, 16.9% by mass at the C2 position, 15.9% by mass at the C3 position, 16.0% by mass at the C4 position, 14.7% by mass at t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com