Method for increasing oxygen flux of fluorite type ion conductor membrane material
A technology of ion conductors and membrane materials, applied in the field of multiphase oxygen-permeable membrane materials, which can solve problems such as reduced reliability, limited oxygen-permeable membrane materials, and power loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Ce 0.5 PR 0.5 o 2-δ +Co 3 o4 Preparation of Multiphase Oxygen Permeable Membrane
[0028] Ce 0.5 PR 0.5 o 2-δ +Co 3 o 4 The preparation of the multiphase oxygen permeable membrane diaphragm is mainly divided into two steps:
[0029] Ce 0.5 PR 0.5 o 2-δ +Co 3 o 4 Synthesis of Multiphase Oxygen Permeable Membrane Materials and Ce 0.5 PR 0.5 o 2-δ +Co 3 o 4 Preparation of Multiphase Oxygen Permeable Membranes.
[0030] Ce 0.5 PR 0.5 o 2-δ +Co 3 o 4 Synthesis of heterogeneous oxygen-permeable membrane materials: Firstly, Ce was synthesized by EDTA-CA sol-gel combined complexation method 0.5 PR 0.5 o 2-δ . Next, weigh a certain mass ratio of Ce 0.5 PR 0.5 o 2-δ Powder and Co 3 o 4 The powder was placed in a ball mill jar (where Co 3 o 4 Powder and Ce 0.5 PR 0.5 o 2-δ The mass percentage of the powder is 20%), and put into a high-energy ball mill (FRITSCH, Pulverisette 6) for ball milling for 3 hours until the mixture is uniform...
Embodiment 2
[0033] Example 2: Ce 0.75 Gd 0.25 o 2-δ +SrCO 3 Preparation of Multiphase Oxygen Permeable Membrane
[0034] Ce 0.75 Gd 0.25 o 2-δ +SrCO 3 The preparation of multiphase oxygen permeable membrane diaphragm is mainly divided into two steps: Ce 0.75 Gd 0.25 o 2-δ +SrCO 3 Synthesis of Multiphase Oxygen Permeable Membrane Materials and Ce 0.75 Gd 0.25 o 2-δ +SrCO 3 Preparation of Multiphase Oxygen Permeable Membranes.
[0035] Ce 0.75 Gd 0.25 o 2-δ +SrCO 3 Synthesis of heterogeneous oxygen-permeable membrane materials: First, Ce was synthesized by hydrothermal synthesis 0.75 Gd 0.25 o 2-δ Material. Next, weigh a certain mass ratio of Ce 0.75 Gd 0.25 o 2-δ Powder and SrCO 3 The powder was placed in a ball mill jar (where SrCO 3 Powder and Ce 0.75 Gd 0.25 o 2-δ The mass percentage of the powder is 10%), and put into a high-energy ball mill (FRITSCH, Pulverisette 6) for ball milling for 5 hours until the mixture is uniform. Then, the slurry was removed a...
Embodiment 3
[0038] Example 3: Ce 0.8 SM 0.2 o 2-δ +SrCO 3 +Co 3 o 4 Preparation of Multiphase Oxygen Permeable Membrane
[0039] Ce 0.8 SM 0.2 o 2-δ +SrCO 3 +Co 3 o 4 The preparation of multiphase oxygen permeable membrane diaphragm is mainly divided into two steps: Ce 0.8 SM 0.2 o 2-δ +SrCO 3 +Co 3 o 4 Synthesis of Multiphase Oxygen Permeable Membrane Materials and Ce 0.8 SM 0.2 o 2- δ +SrCO 3 +Co 3 o 4 Preparation of Multiphase Oxygen Permeable Membranes.
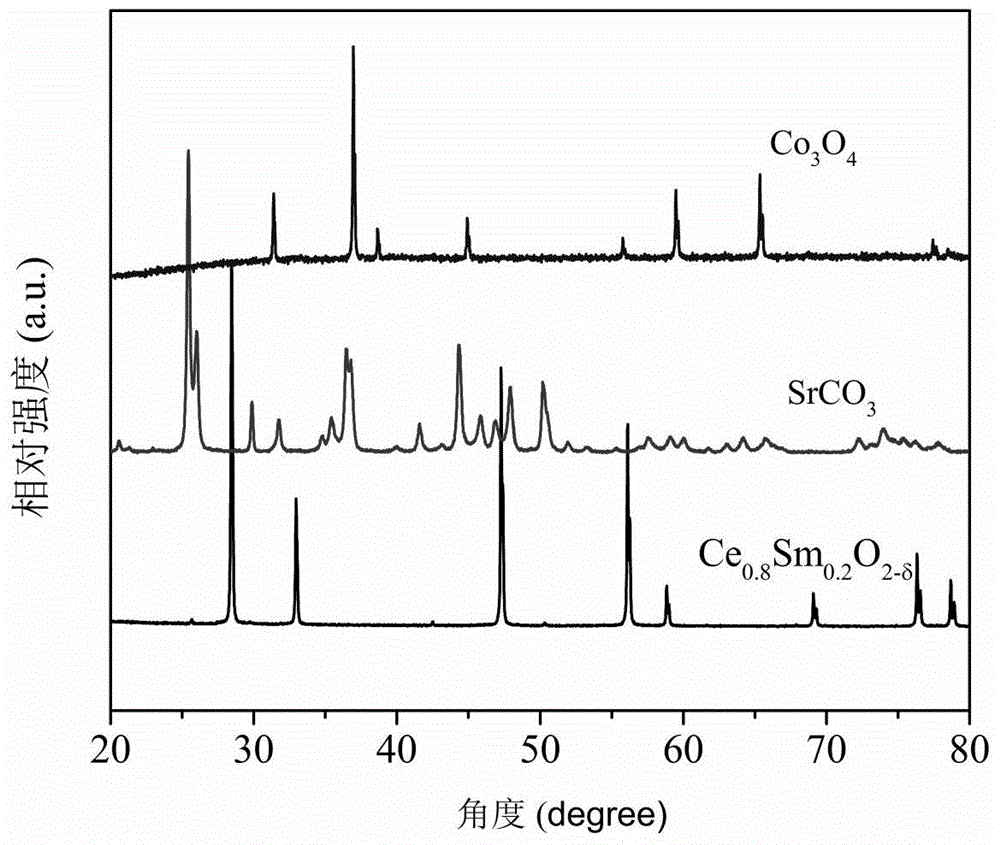
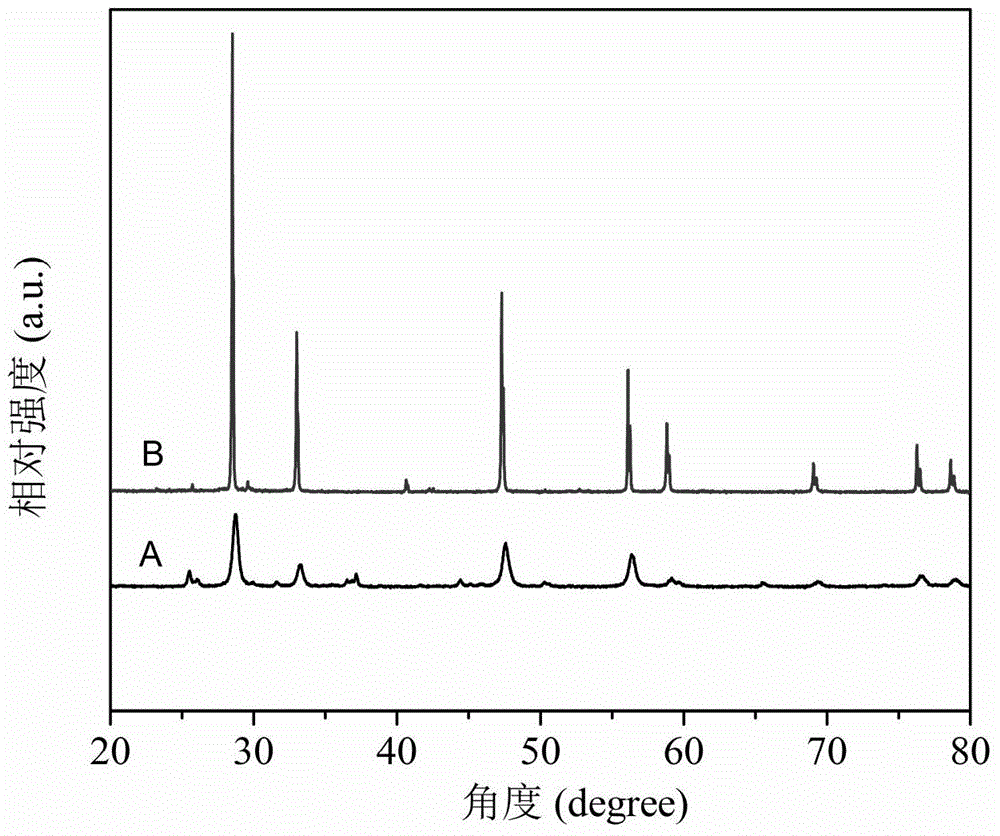
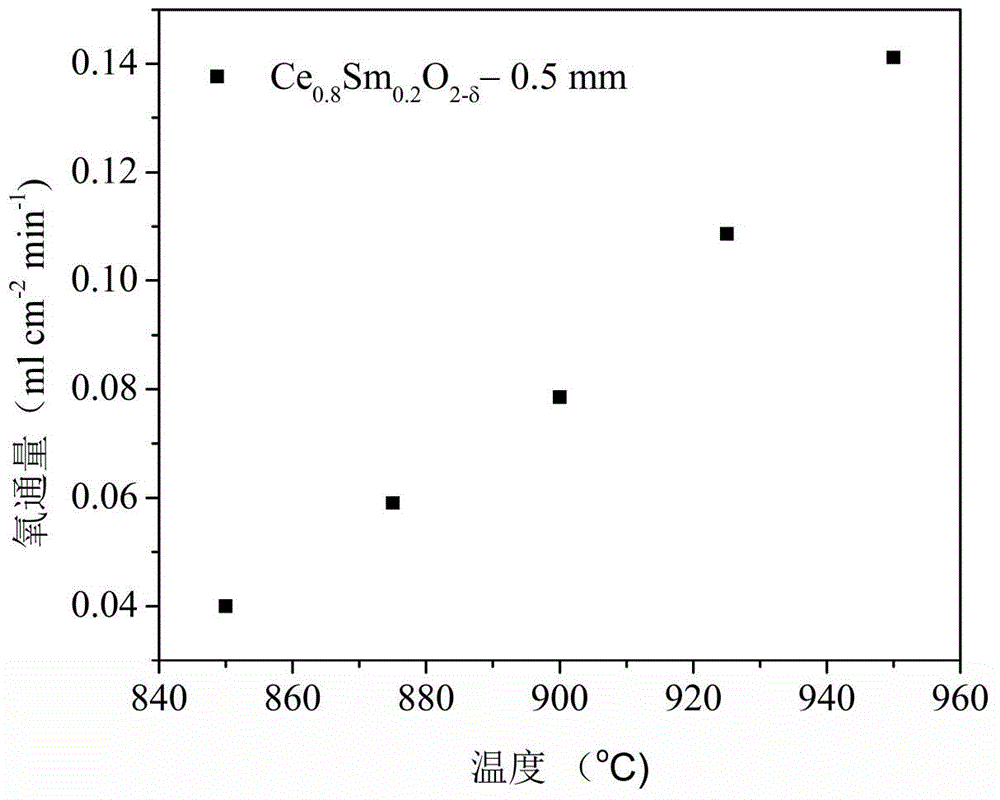
[0040] Ce 0.8 SM 0.2 o 2-δ +SrCO 3 +Co 3 o 4 Synthesis of heterogeneous oxygen-permeable membrane materials: First, Ce was synthesized by hydrothermal synthesis 0.5 PR 0.5 o 2-δ Material. Next, weigh a certain mass ratio of Ce 0.8 SM 0.2 o 2-δ Powder, SrCO 3 Powder and Co 3 o 4 Powder (Ce 0.8 SM 0.2 o 2-δ , SrCO 3 ,Co 3 o 4 The X-ray diffraction curve of figure 1 shown) placed in the ball mill tank (in which SrCO 3 Powder and Co 3 o 4 Total powder and Ce 0.8 SM 0.2 o 2-δ The mass ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com