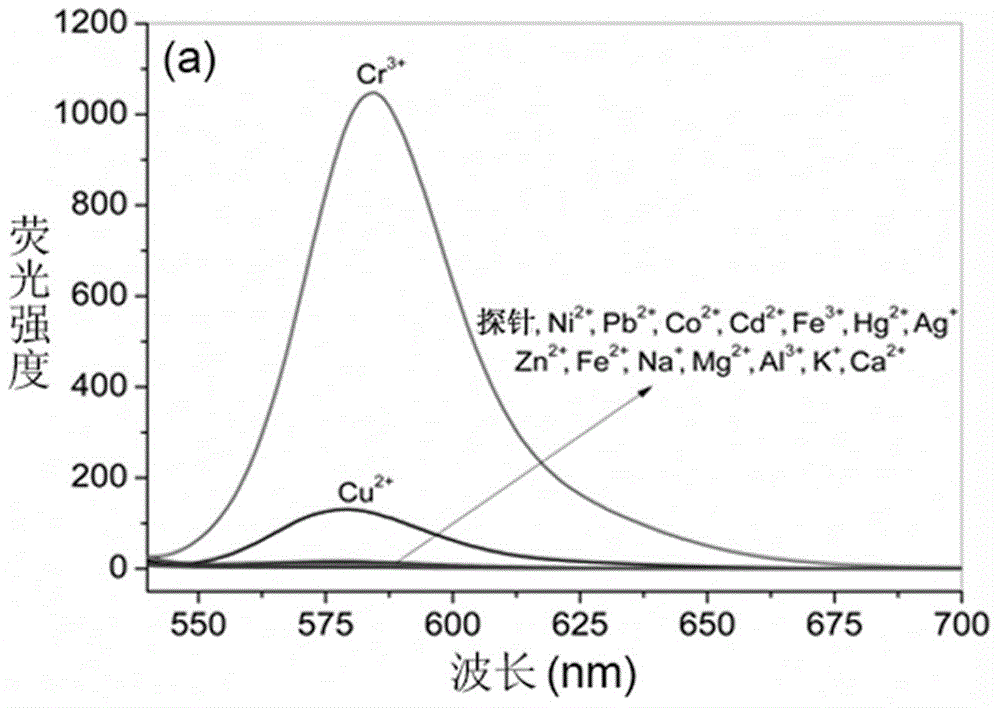
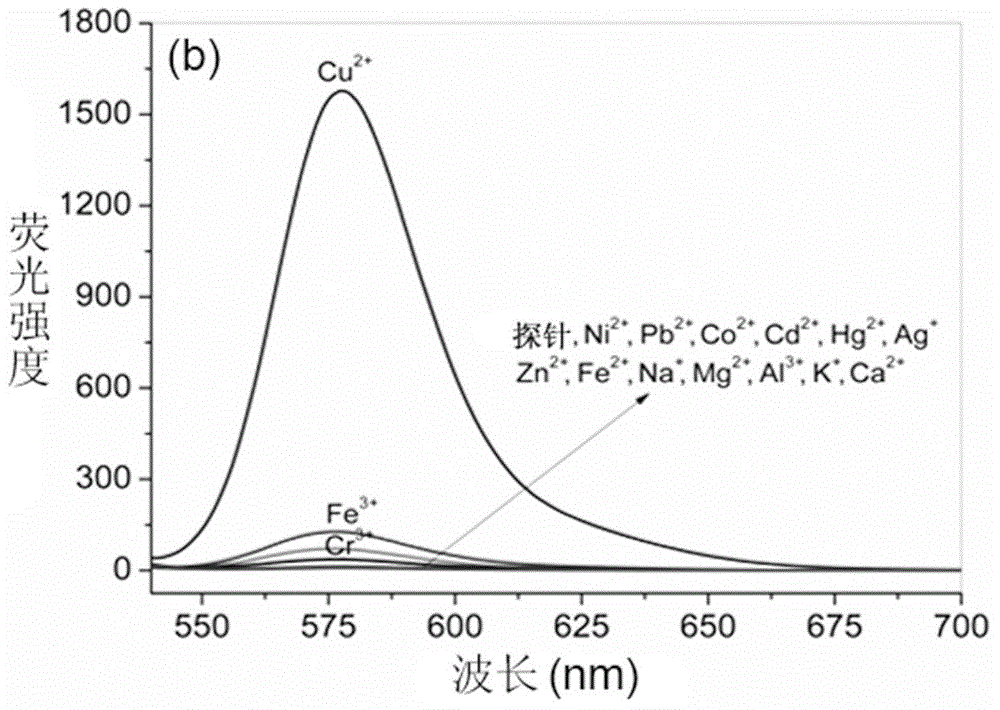
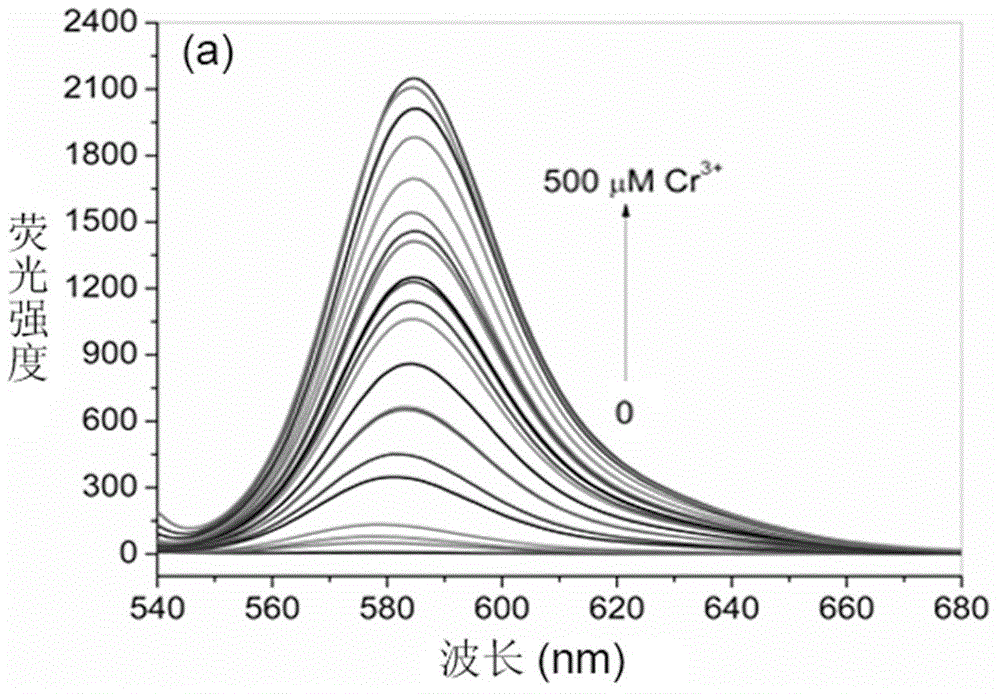
Rhodamine B derivative containing 8-aminoquinoline group, preparation method, application and method for carrying out fluorescence analysis on Cr<3+> and Cu<2+>
A technology for aminoquinoline and fluorescence analysis, which is applied in the direction of material analysis, material analysis, fluorescence/phosphorescence, etc. through optical means, can solve problems such as single action and function, complex synthesis process, etc., and achieve high sensitivity and wide application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation method of the rhodamine B derivative containing 8-aminoquinoline group of the present invention comprises the following steps:
[0039] 1) Take a certain amount of N-bromoethylrhodamine B lactam, 8-aminoquinoline and acetonitrile, then dissolve N-bromoethylrhodamine B lactam and 8-aminoquinoline in acetonitrile, and then Then add triethylamine to obtain solution A, wherein the ratio of N-bromoethylrhodamine B-lactam, 8-aminoquinoline, triethylamine and acetonitrile is 100 mg: 50 mg: 100 μL: 10 mL;
[0040] 2) Protect the solution A obtained in step 1) with nitrogen, and stir the reaction under reflux conditions. After the reaction is completed, cool to room temperature, remove the solvent, and then elute through column chromatography to obtain a light yellow solid It is a rhodamine B derivative containing 8-aminoquinoline group.
[0041] Step 2) stirring 70h reaction under the condition of reflux;
[0042] The specific process of column chromatography ...
Embodiment 2
[0044] The preparation method of the rhodamine B derivative containing 8-aminoquinoline group of the present invention comprises the following steps:
[0045] 1) Take a certain amount of N-bromoethylrhodamine B lactam, 8-aminoquinoline and acetonitrile, then dissolve N-bromoethylrhodamine B lactam and 8-aminoquinoline in acetonitrile, and then Then add triethylamine to obtain solution A, wherein the ratio of N-bromoethylrhodamine B lactam, 8-aminoquinoline, triethylamine and acetonitrile is 100mg: 100mg: 50 μL: 15mL;
[0046] 2) Protect the solution A obtained in step 1) with nitrogen, and stir the reaction under reflux conditions. After the reaction is completed, cool to room temperature, remove the solvent, and then elute through column chromatography to obtain a light yellow solid It is a rhodamine B derivative containing 8-aminoquinoline group.
[0047] Step 2) stirring 60h reaction under the condition of reflux;
[0048] The specific process of column chromatography elu...
Embodiment 3
[0050] The preparation method of the rhodamine B derivative containing 8-aminoquinoline group of the present invention comprises the following steps:
[0051] 1) Take a certain amount of N-bromoethylrhodamine B lactam, 8-aminoquinoline and acetonitrile, then dissolve N-bromoethylrhodamine B lactam and 8-aminoquinoline in acetonitrile, and then Then add triethylamine to obtain solution A, wherein the ratio of N-bromoethylrhodamine B lactam, 8-aminoquinoline, triethylamine and acetonitrile is 100 mg: 70 mg: 80 μL: 14 mL;
[0052] 2) Protect the solution A obtained in step 1) with nitrogen, and stir the reaction under reflux conditions. After the reaction is completed, cool to room temperature, remove the solvent, and then elute through column chromatography to obtain a light yellow solid It is a rhodamine B derivative containing 8-aminoquinoline group.
[0053] Step 2) stirring the reaction for 55h under the condition of reflux;
[0054] The specific process of column chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com