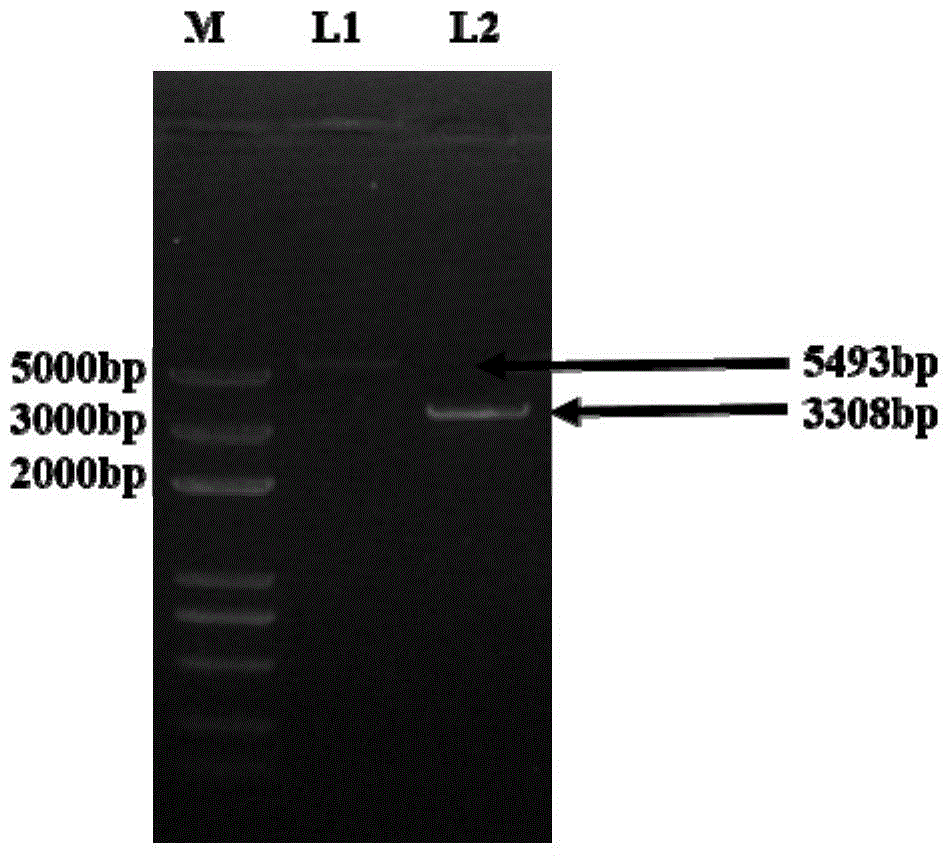
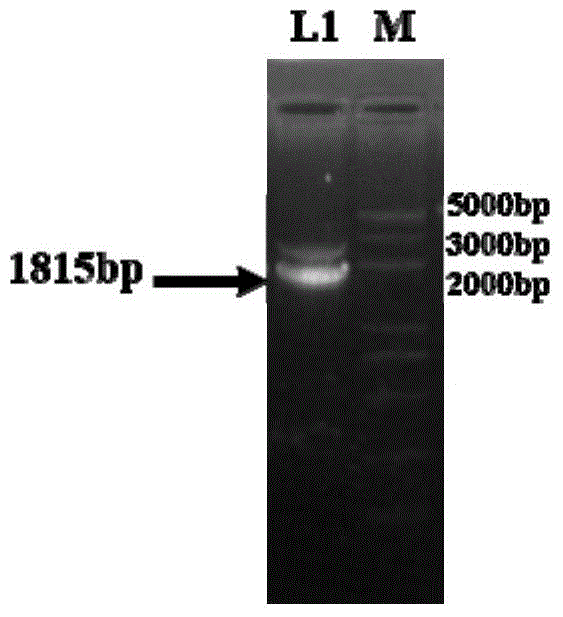
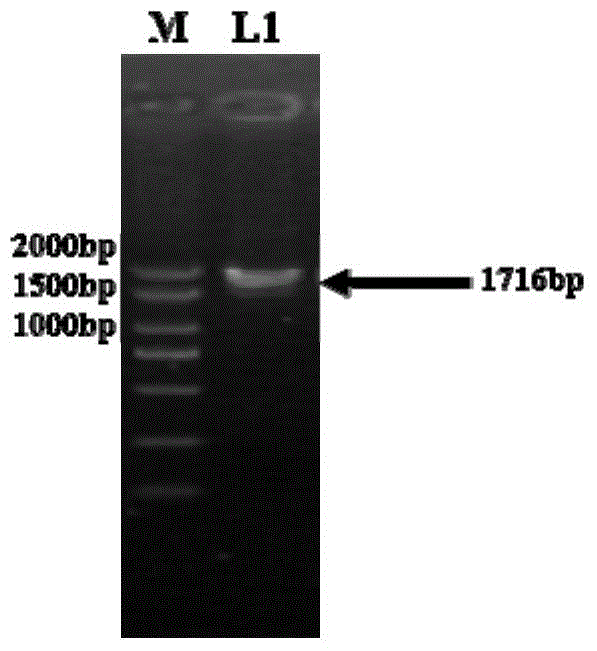
Method for improving enzyme activity of trehalose synthase by C-terminal fragments of thermophilic bacteria trehalose synthase
A technology of trehalose synthase and thermophilic bacteria, applied in the field of enzyme genes, can solve problems such as improving trehalose production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 Cloning of the coding region of the trehalose synthase gene derived from Pseudomonas putida, Corynebacterium glutamicum, Streptomyces coelicolor and Thermotoga maritima and its fusion expression in Escherichia coli
[0089] 1. Primer design and PCR reaction
[0090] Pseudomonas putida NBRC14164, Corynebacterium glutamicum ATCC 13032, Streptomyces coelicolor ATCC 23899, Thermotoga marine MSB8 and Thermus thermophilus) HB27 genome was provided by Yan Yajun, a professor at the University of Georgia in the United States, and these strains can be purchased from the China General Microorganism Culture Collection Center.
[0091] According to the known coding sequence of trehalose synthase gene from four sources of Pseudomonas putida, Corynebacterium glutamicum, Streptomyces coelicolor and Thermotoga maritima, a pair of upstream and downstream primers were designed respectively. for:
[0092] The upstream primer of the trehalose synthase gene fragment derived from ...
Embodiment 2
[0145] Example 2 The C-terminal thermophilic fragment gene coding region of thermophilic bacteria trehalose synthase derived from Pseudomonas putida, Corynebacterium glutamicum, Streptomyces coelicolor and Thermotoga maritima fused to it Cloning and fusion expression in Escherichia coli
[0146] 1. Primer design and PCR reaction
[0147] Pseudomonas putida NBRC14164, Corynebacterium glutamicum ATCC 13032, Streptomyces coelicolor ATCC 23899, Thermotoga marine MSB8 and Thermus thermophilus) HB27 genome was provided by Yan Yajun, a professor at the University of Georgia in the United States, and these strains can be purchased from China General Microorganism Culture Collection Center.
[0148] According to the sequence of the C-terminal thermophilic fragment coding region of trehalose synthase gene from known thermophilic bacteria and seaweed from four sources: Pseudomonas putida, Corynebacterium glutamicum, Streptomyces coelicolor and Thermotoga maritima Sugar synthase gene co...
Embodiment 3
[0224] Example 3 Effect of C-terminal fragment of trehalose synthase derived from thermophilic bacteria on enzymatic activity of trehalose synthase derived from Pseudomonas putida, Corynebacterium glutamicum, Streptomyces coelicolor and Thermotoga maritima
[0225] The enzymatic activity and activity of four trehalose synthases derived from Pseudomonas putida, Corynebacterium glutamicum, Streptomyces coelicolor and Thermotoga maritima by adding the C-terminal gene fragment of trehalose synthase derived from thermophilic bacteria Compared with the original trehalose synthase, it was increased by 2.53 times, 5.58 times, 2.74 times and 4.57 times respectively. Therefore, it is proved that the trehalose synthase derived from thermophilic bacteria does have a certain impact on the catalytic properties of the other four sources of trehalose synthase, and plays a crucial role.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com