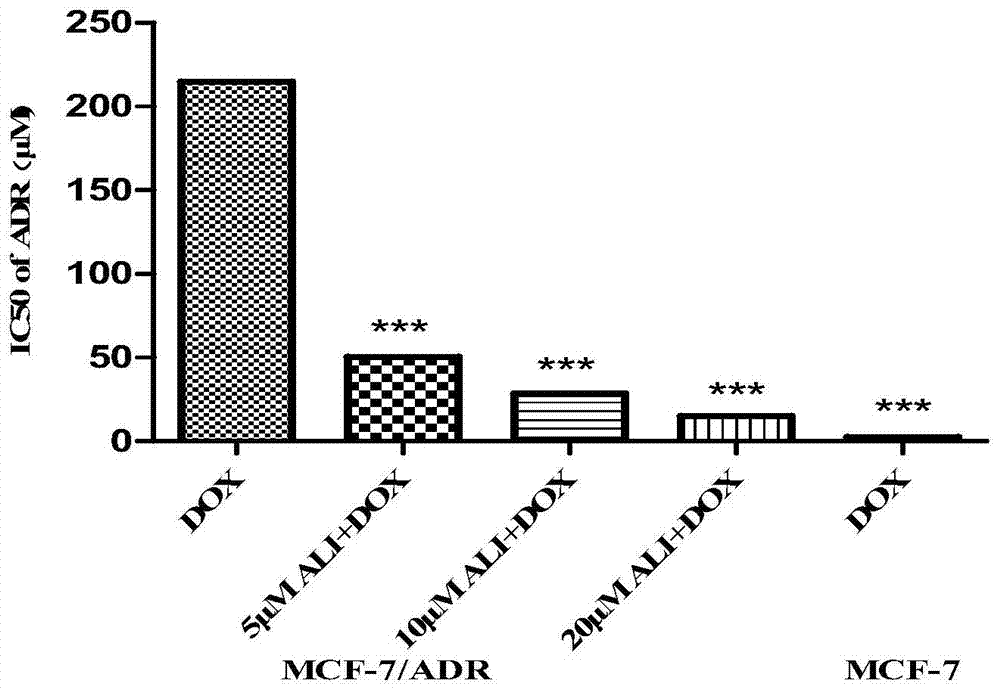
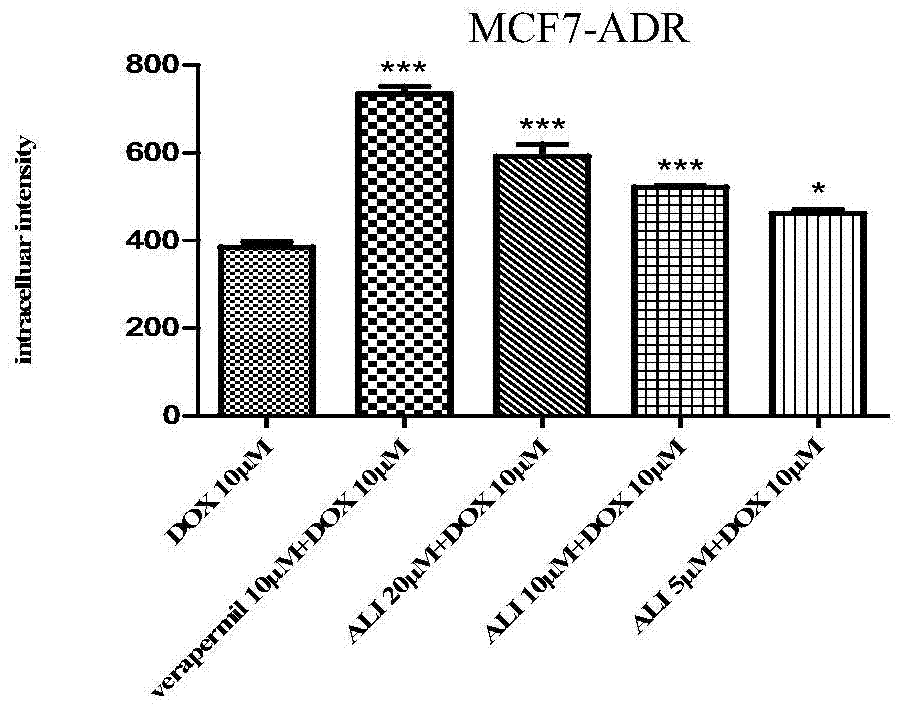
New uses of 24‑acetyl alisol f
A technology for the use of acetyl alisol, which is applied in the field of proterpane-type tetracyclic triterpenoids, can solve the problems that the pharmacological activity has not been reported, and achieve the effects of promoting nuclear entry, promoting early apoptosis, and increasing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] An antitumor pharmaceutical composition is composed of 24-acetyl alisol F and doxorubicin, and the molar concentration ratio of the 24-acetyl alisol F and doxorubicin is 1:1.
[0040] The preparation method of above-mentioned pharmaceutical composition, concrete steps are as follows:
[0041] (1) Take by weighing 24-acetyl alisol F and doxorubicin respectively, for subsequent use;
[0042] (2) Add DMSO (dimethyl sulfoxide) solvent to dissolve 24-acetyl alisol F, and obtain 24-acetyl alisol F mother liquor;
[0043] (3) add ultrapure water to dissolve adriamycin to obtain a doxorubicin mother liquor;
[0044] (4) Take RPMI1640 basal culture, dilute the 24-acetyl alisol F mother liquor step by step to obtain 24-acetyl alisitol F solutions with molar concentrations of 5, 10, and 20 μM;
[0045] (5) Take RPMI1640 basal culture, dilute the doxorubicin mother liquor step by step to obtain a doxorubicin solution with a molar concentration of 10 μM;
[0046] (6) Take 24-acet...
Embodiment 2
[0048] An antitumor pharmaceutical composition is composed of 24-acetyl alisol F and doxorubicin, and the molar concentration ratio of the 24-acetyl alisol F and doxorubicin is 0.5:1.
[0049]The preparation method of above-mentioned pharmaceutical composition, concrete steps are as follows:
[0050] (1) Take by weighing 24-acetyl alisol F and doxorubicin respectively, for subsequent use;
[0051] (2) Add DMSO (dimethyl sulfoxide) solvent to dissolve 24-acetyl alisol F, and obtain 24-acetyl alisol F mother liquor;
[0052] (3) add ultrapure water to dissolve adriamycin to obtain a doxorubicin mother liquor;
[0053] (4) Take RPMI1640 basal culture, dilute the 24-acetyl alisol F mother liquor step by step to obtain 24-acetyl alisitol F solutions with molar concentrations of 5, 10, and 20 μM;
[0054] (5) Take RPMI1640 basal culture, dilute the doxorubicin mother liquor step by step to obtain a doxorubicin solution with a molar concentration of 10 μM;
[0055] (6) Take 24-ace...
Embodiment 3
[0057] An antitumor pharmaceutical composition is composed of 24-acetyl alisol F and doxorubicin, and the molar concentration ratio of the 24-acetyl alisol F and doxorubicin is 2:1.
[0058] The preparation method of above-mentioned pharmaceutical composition, concrete steps are as follows:
[0059] (1) Take by weighing 24-acetyl alisol F and doxorubicin respectively, for subsequent use;
[0060] (2) Add DMSO (dimethyl sulfoxide) solvent to dissolve 24-acetyl alisol F, and obtain 24-acetyl alisol F mother liquor;
[0061] (3) add ultrapure water to dissolve adriamycin to obtain a doxorubicin mother liquor;
[0062] (4) Take RPMI1640 basal culture, dilute the 24-acetyl alisol F mother liquor step by step to obtain 24-acetyl alisitol F solutions with molar concentrations of 5, 10, and 20 μM;
[0063] (5) Take RPMI1640 basal culture, dilute the doxorubicin mother liquor step by step to obtain a doxorubicin solution with a molar concentration of 10 μM;
[0064] (6) Take 24-acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com