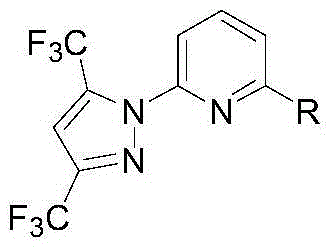
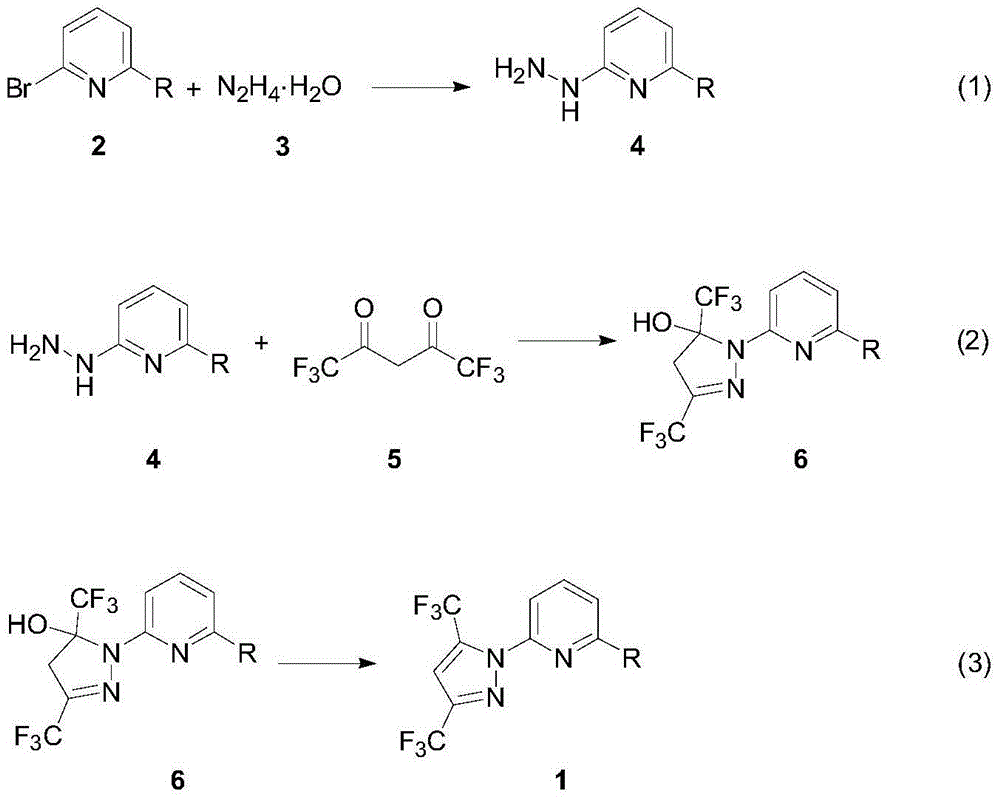
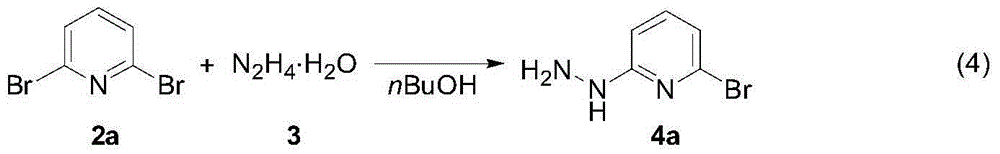Synthesis method of (3,5-bistrifluoromethylpyrazolyl)pyridine derivatives
A technology of trifluoromethylpyrazolyl and dimethylpyrazolyl, which is applied in the field of preparation of pyridine derivatives, can solve problems such as the weakening of pyrazole nucleophilicity, and achieve easy derivatization, easy preparation, and wide range of product uses Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] A mixture of 2,6-dibromopyridine 2a (2.37g, 10.0mmol), hydrazine hydrate (20.0mmol) and 20mL n-butanol was stirred and reacted at 120°C for 12 hours. After cooling to room temperature, the volatile components were removed under reduced pressure. The solid was washed three times with water (5mL) and ether (5mL), and dried under vacuum at 50℃ for 2 hours to obtain brown solid 4a as the target product (1.25g, yield 67%) . The target product was confirmed by nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry.
Embodiment 2
[0029]
[0030] The reaction steps and operation are the same as in Example 1, and the difference from Example 1 is that 2-bromo-6-(3,5-dimethylpyrazolyl)pyridine (2b) (2.51g, 10.0mmol). After stopping the reaction, the brown solid 4b was obtained as the target product (1.30 g, yield 65%) after the same post-treatment. The target product was confirmed by nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry. Compound 2b was prepared according to literature methods (Sun, X.J.; Yu, Z.K.; Wu, S.Z.; Xiao, W.J. Organometallics 2005, 24, 2959).
Embodiment 3
[0032]
[0033] In a 25mL Schlenk reaction flask, add 2-bromo-6-hydrazine pyridine 4a (187mg, 1.0mmol), hexafluoroacetylacetone (242mg, 1.2mmol), trifluoroacetic acid (50μL) and 15mL tetrahydrofuran in sequence, and stir to react under reflux for 5 hour. After the reaction, the mixture was cooled to room temperature, the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent: petroleum ether (60-90°C) / diethyl ether, v / v=50:1), A white solid target product 6a (346 mg, yield 94%) was obtained. The target product was confirmed by nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com