Quality control method for kudzuvine root and hawthorn lipid-lowering particles
A quality control method, Geshan lipid-lowering technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of complex sample preparation for puerarin content determination, difficulty in reflecting the monitoring requirements of the compatibility characteristics of this prescription and the need for rapid inspection, etc. To achieve the effect of comprehensive quality supervision, ensuring stability and accurate test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The technical solution of the present invention is described in detail below through the embodiments and accompanying drawings, and the illustrated embodiments and accompanying drawings do not limit the present invention.
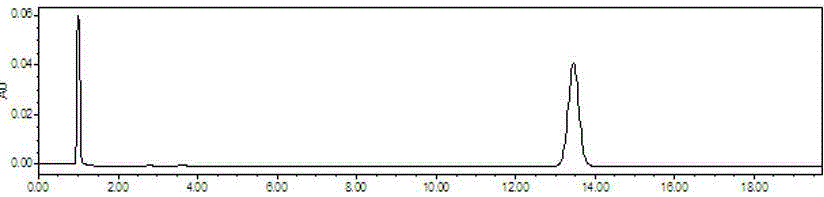
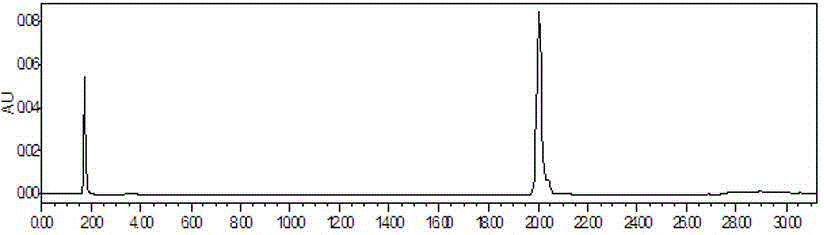
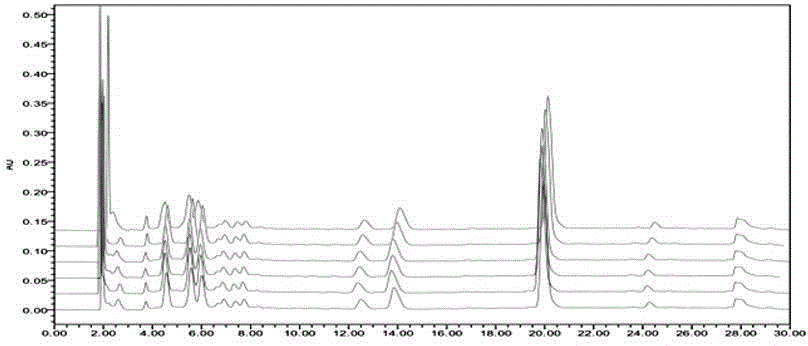
[0048] 1. The method for determining the content of puerarin is:
[0049] 1.1 Samples, instruments and reagents.
[0050] Geshan Jiangzhi Granules (prepared according to the technology of Geshan Jiangzhi Granules of Lvyin Pharmaceutical Co., Ltd.; batch numbers 20120319, 20120325, 20120401, 20120407, 20120411, 20120413, 20120417, 20120420, 20120424, 20120430), puerarin reference substance (China Institute for the Control of Pharmaceutical and Biological Products ).
[0051] Waters1525 high performance liquid chromatograph, Waters2489 detector, SHIMADZU AUW120D one hundred thousandth balance, XMTD-4000 constant temperature water bath (Beijing Yongming Medical Instrument Co., Ltd.); TU-1810 ultraviolet-visible spectrophotometer.
[0052] Chromatograp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com