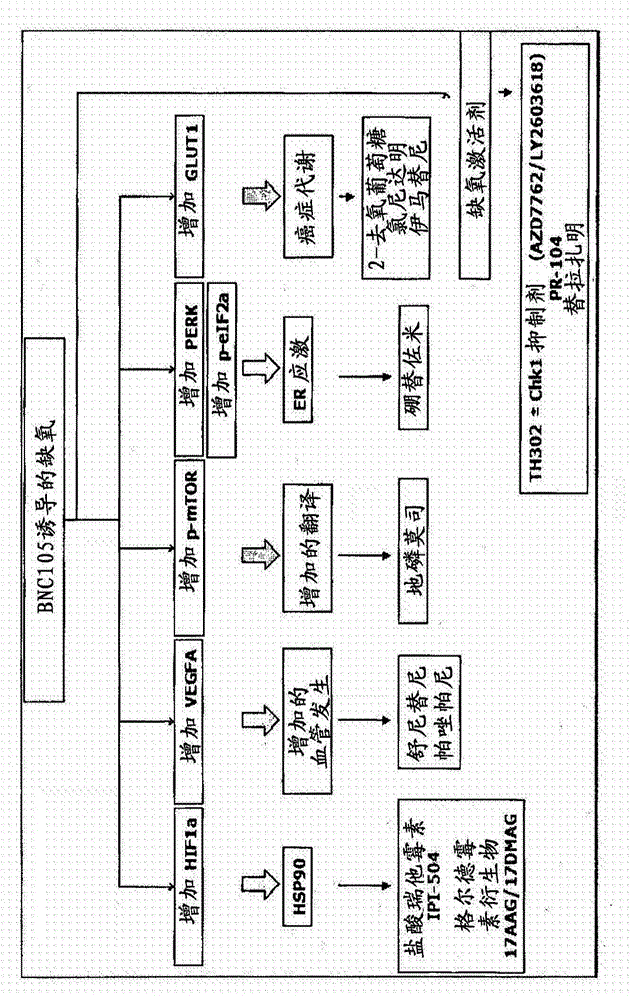
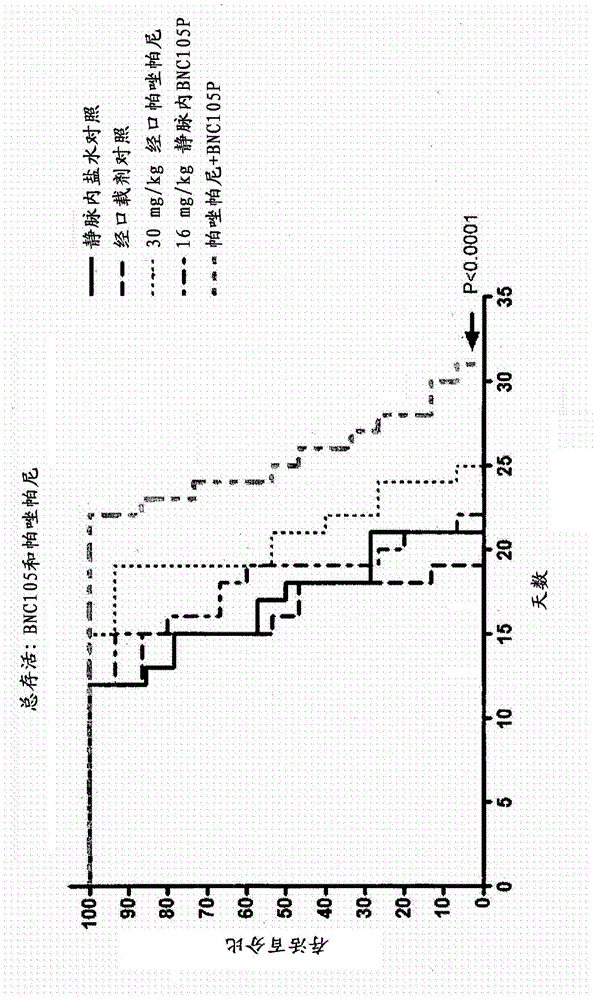
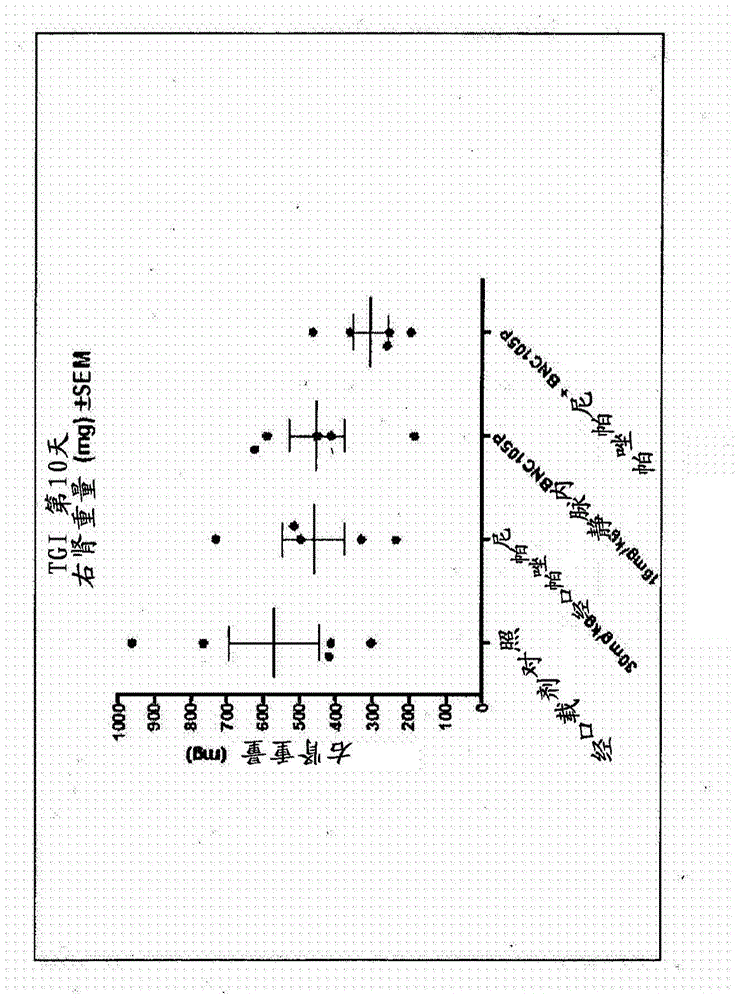
Combination therapy involving a vascular disrupting agent and an agent which targets hypoxia
A technology of vascular disruptor and targeting agent, applied in the field of cancer treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241] Preparation of 2-methyl-7-hydroxy-3-(3,4,5-trimethoxybenzoyl)-6-methoxybenzofuran (BNC105)
[0242]
[0243] Preparation A
[0244] To 2-bromo-7-acetoxy-3-(3,4,5-trimethoxybenzoyl)-6-methanol in 1,4-dioxane (2 mL) at 90°C A stirred solution of oxybenzofuran (20 mg, 0.042 mmol), methylboronic acid (40 mg, 0.67 mmol) was added tetrakis-triphenylphosphine palladium (11 mg, 0.01 mmol), followed by carbonic acid in distilled water (0.5 mL) Sodium hydrogen (40mg, 0.48mmol) solution. After 5 minutes, the reaction mixture turned red. After 2 hours (tlc), the reaction mixture was warmed to room temperature and saturated ammonium chloride (2 mL) was added and diluted with dichloromethane (20 mL). The organic layer was separated and washed with water, dried over magnesium sulfate and the solvent was removed by distillation under vacuum. The residue was purified by PTLC (eluent=dichloromethane / methanol, 1:1) to give the title compound as a fluffy white solid (acetate cleav...
Embodiment 2
[0249] Preparation of 6-methoxy-2-methyl-3-(3,4,5-trimethoxybenzoyl)benzofuran-7-yl phosphate disodium ester
[0250]
[0251] Step 1: Dibenzyl 6-methoxy-2-methyl-3-(3,4,5-trimethoxybenzoyl)benzofuran-7-ylphosphate:
[0252]To 0.081 g (0.22 mmol) (7-hydroxy-6-methoxy-2-methylbenzofuran-3-yl) (3,4 , 5-trimethoxyphenyl)methanone, a mixture of 0.086 g (0.261 mmol) carbon tetrabromide and 0.063 mL (0.283 mmol) dibenzyl phosphite was added dropwise with 0.046 mL of anhydrous triethylamine. The resulting mixture was stirred at room temperature for 2 hours, then diluted to 20ml with ethyl acetate, washed with brine, dried over anhydrous magnesium sulfate, filtered and evaporated to dryness under reduced pressure. The residue was purified by flash column chromatography (dichloromethane / ethyl acetate, 9:1) to give the title compound as a colorless foam, (0.13 g, 94%);
[0253] 1 H NMR (CDCl 3 )δ2.42(s, 3H, Me-2); 3.83(s, 1H, OMe); 3.93(s, 3H, OMe); 5.33(m, 4H, CH 2 Ph); 6.89 (d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com