Catalyst for preparation of aldehyde through heterogeneous catalysis of fat primary alcohol dehydrogenation
A heterogeneous catalysis and catalyst technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, preparation of carbon-based compounds, etc. There are 17% and other problems, to achieve the effect of high selectivity, simple recycling and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
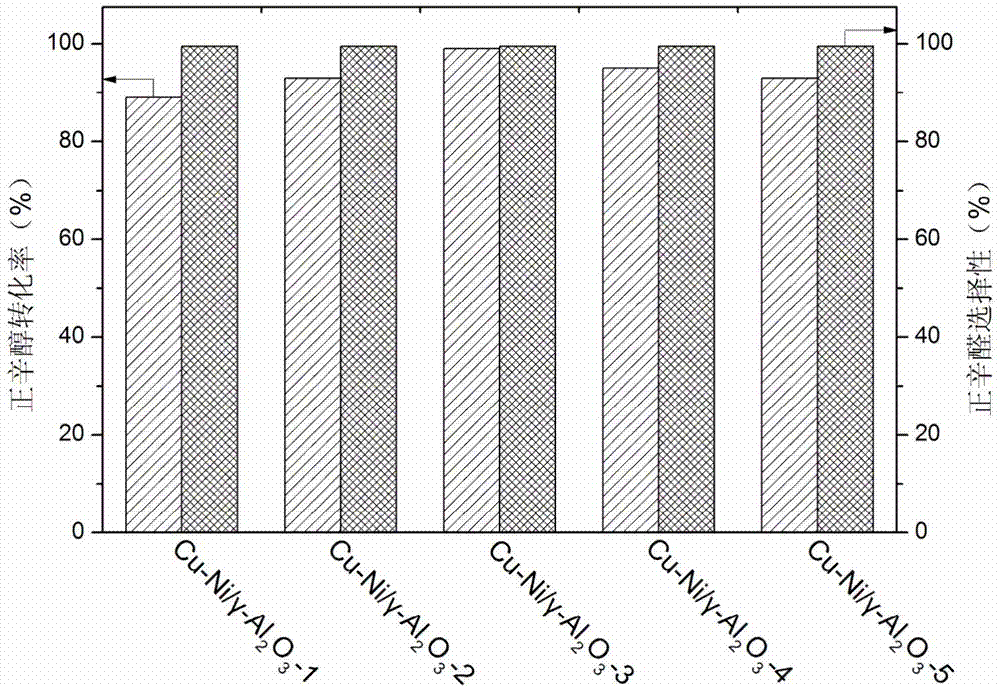
[0020] Example 1: Cu-Ni / γ-Al 2 o 3 Preparation of a series of catalysts
[0021] According to the mass content of Cu shown in Table 1, a copper nitrate solution with a suitable concentration was prepared, and the pH of the copper nitrate solution was adjusted to 9 with ammonia water. Put the above solution in an ice-water bath under stirring conditions, disperse 2g of activated alumina in the copper nitrate solution and stir for 3h, then dilute with a large amount of water, finally, filter and wash three times, dry at 80°C for 10h, and roast at 400°C for 6h , to obtain the catalyst precursor Cu / γ-Al 2 o 3 .
[0022] According to the mass ratio of Cu and Ni shown in Table 1, prepare a nickel nitrate solution with a suitable concentration, take 2g Cu / γ-Al 2 o 3 Add it and stir for 3h, dry at 80°C for 10h, and bake at 400°C for 6h. The obtained catalyst was reduced in a hydrogen atmosphere at 500° C. for 5 h before use.
[0023] Table 1
[0024]
Embodiment 2
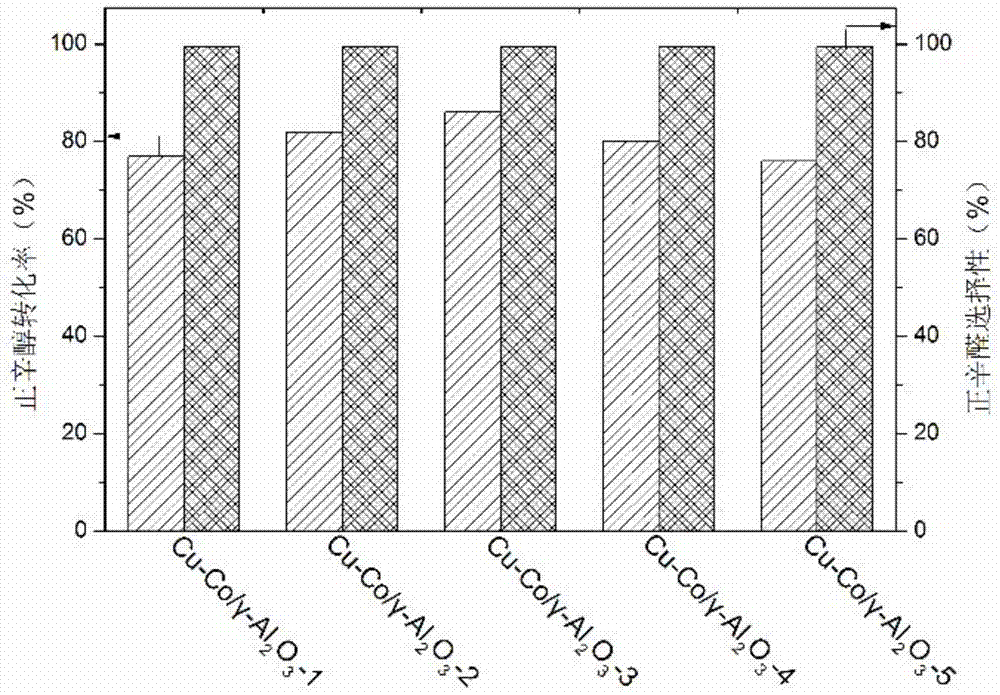
[0025] Example 2: Cu-Co / γ-Al 2 o 3 Preparation of a series of catalysts
[0026] According to the mass content of Cu shown in Table 2, a copper nitrate solution with a suitable concentration was prepared, and the pH of the copper nitrate solution was adjusted to 9 with ammonia water. Put the above solution in an ice-water bath under stirring conditions, disperse 2g of activated alumina in the copper nitrate solution and stir for 3h, then dilute with a large amount of water, finally, filter and wash three times, dry at 80°C for 10h, and roast at 400°C for 6h , to obtain the catalyst precursor Cu / γ-Al 2 o 3 .
[0027] According to the mass ratio of Cu and Co shown in Table 2, prepare a cobalt nitrate solution with a suitable concentration, take 2g Cu / γ-Al 2 o 3 Add it and stir for 3h, dry at 80°C for 10h, and bake at 400°C for 6h. The obtained catalyst was reduced in a hydrogen atmosphere at 500° C. for 5 h before use.
[0028] Table 2
[0029]
Embodiment 3
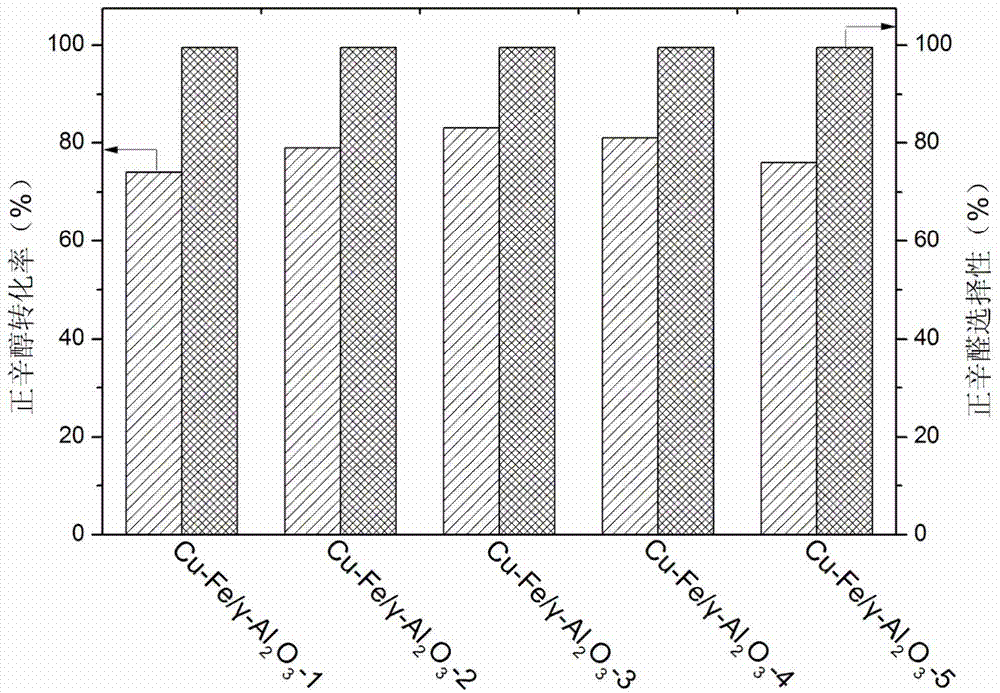
[0030] Example 3: Cu-Mn / γ-Al 2 o 3 Preparation of a series of catalysts
[0031] According to the mass content of Cu shown in Table 3, a copper nitrate solution with a suitable concentration was prepared, and the pH of the copper nitrate solution was adjusted to 9 with ammonia water. Put the above solution in an ice-water bath under stirring conditions, disperse 2g of activated alumina in the copper nitrate solution and stir for 3h, then dilute with a large amount of water, finally, filter and wash three times, dry at 80°C for 10h, and roast at 400°C for 6h , to obtain the catalyst precursor Cu / γ-Al 2 o 3 .
[0032] According to the mass ratio of Cu and Mn shown in Table 3, prepare a manganese nitrate solution with a suitable concentration, take 2g Cu / γ-Al 2 o 3 Add it and stir for 3h, dry at 80°C for 10h, and bake at 400°C for 6h. The obtained catalyst was reduced in a hydrogen atmosphere at 500° C. for 5 h before use.
[0033] table 3
[0034]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap