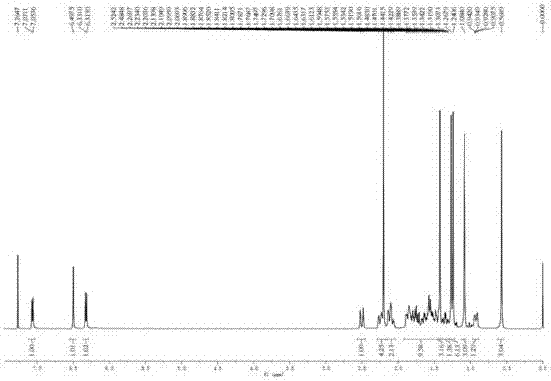
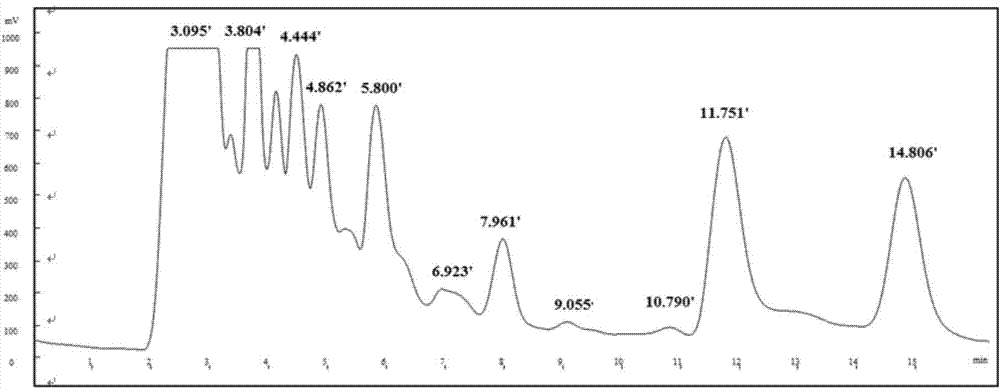
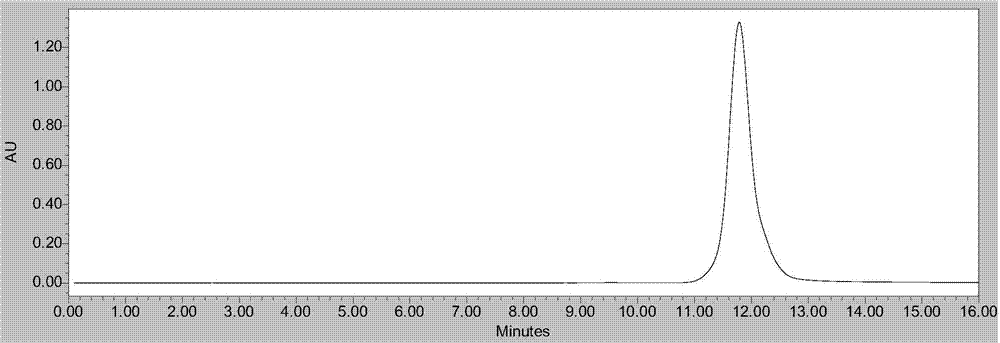
Method for rapidly preparing tripterine chemical reference substance from Common Threewingnut Root extract
A technology of tripterygenin chemistry and tripterygenin, which is applied in the field of rapid preparation of tripterygenin chemical reference substances from tripterygium wilfordii extract, can solve the problems of small preparation flux and unguaranteed purity, and achieve rapid Effects of recycling, improving purity, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Ethyl acetate extraction
[0030] 500g of the root of Tripterygium wilfordii, extracted 3 times with 1500mL of absolute ethanol, 3 hours each time, combined the three extracts, recovered under reduced pressure to obtain about 31g of the total extract, dispersed with about 120mL of water, and extracted 3 times with ethyl acetate , about 150mL each time. The extracts were combined and recovered under reduced pressure to obtain about 15 g of ethyl acetate.
[0031] 2) Separation by silica gel column chromatography
[0032] Add about 15g of silica gel to mix well, dry under reduced pressure, and separate by silica gel vacuum chromatography (40cm*5cm), use 5 times column volume of petroleum ether: ethyl acetate (volume ratio 4:1) ), petroleum ether: ethyl acetate (volume ratio 2:1), petroleum ether: ethyl acetate (volume ratio 1:1) step gradient elution, thin-layer chromatography detection, with petroleum ether: ethyl acetate (volume ratio 2 : 1) as developer, GF254 fl...
Embodiment 2
[0038] 1) Ethyl acetate extraction
[0039] 1000g of tripterygium twig root, extracted once with 3000mL of 80% ethanol / water for 3 hours, recovered under reduced pressure to obtain about 52g of total extract, dispersed with about 250mL of water, and extracted once with ethyl acetate, about Use 300mL. Recover under reduced pressure to obtain about 26 g of ethyl acetate.
[0040] 2) Separation by silica gel column chromatography
[0041] Add about 26g of silica gel to mix well, dry under reduced pressure, and separate by silica gel decompression chromatography (45cm*6cm), and use 5 times the column volume of petroleum ether: ethyl acetate (volume ratio 4:1) ), petroleum ether: ethyl acetate (volume ratio 2:1), petroleum ether: ethyl acetate (volume ratio 1:1) step gradient elution, thin-layer chromatography detection, with petroleum ether: ethyl acetate (volume ratio 2 : 1) as developer, GF254 fluorescent plate, dark spots were inspected at 254nm of ultraviolet lamp, the elua...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com