Cyanide-free gold nickel alloy plating electroplate liquid
A cyanide-free gold plating and nickel alloy technology, applied in the field of gold-nickel alloy liquid, can solve the problems of high toxicity, high cost, unstable cyanide-free gold-nickel alloy liquid, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] When using the following cyanide-free gold-plated nickel alloy electroplating solution for electroplating, select the copper electrode as the cathode and the carbon plate as the anode. Adjust the temperature to a predetermined value at a current density of 1A / dm 2 ~5A / dm 2 Under plating.
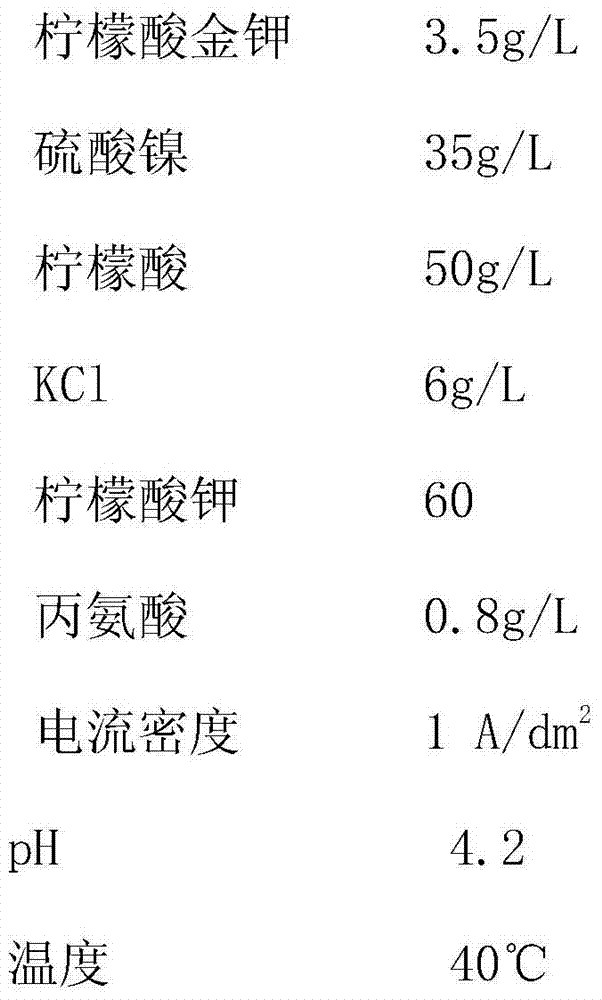
[0022] The composition of the gold-plated nickel alloy electroplating solution is as follows:
[0023]
[0024] According to the electroplating step in the specific embodiment, the electroplating operation is performed using the cyanide-free gold-plated nickel alloy electroplating solution. Results The copper electrode was plated with gold-nickel alloy, and the weight percentage of gold was 95.83% (23K gold) through analysis and test.
Embodiment 2
[0026] When using the following cyanide-free gold-plated nickel alloy electroplating solution for electroplating, select the copper electrode as the cathode and the carbon plate as the anode. Adjust the temperature to a predetermined value at a current density of 1A / dm 2 ~5A / dm 2 Under plating.
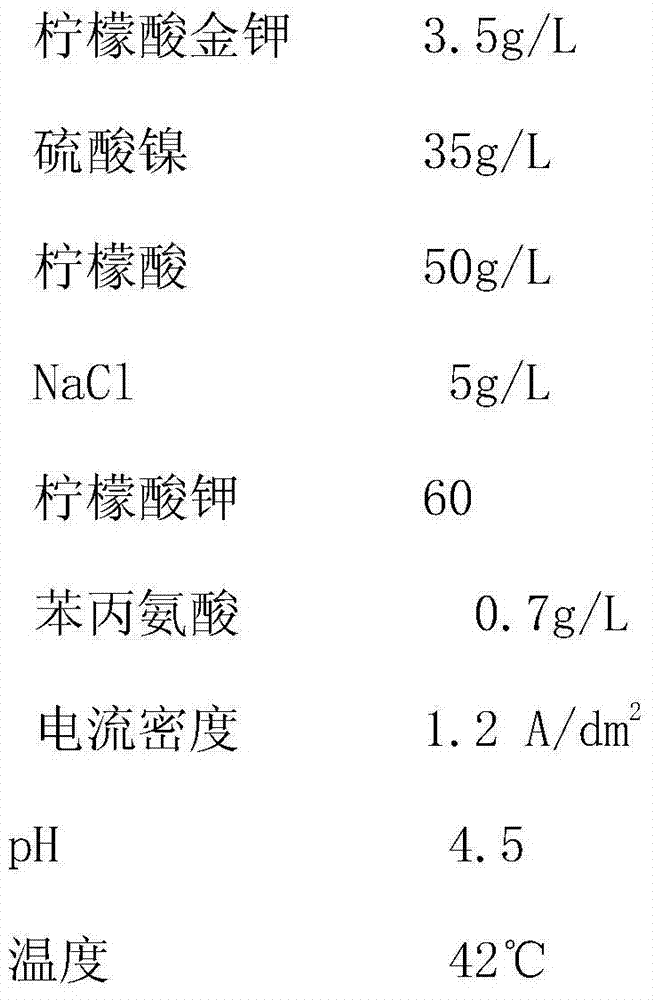
[0027] The composition of the gold-plated nickel alloy electroplating solution is as follows:
[0028]
[0029] According to the electroplating step in the specific embodiment, the electroplating operation is performed using the cyanide-free gold-plated nickel alloy electroplating solution. Results The copper electrode was plated with gold-nickel alloy, and the weight percentage of gold was 95.83% (23K gold) through analysis and test.
Embodiment 3
[0031] When using the following cyanide-free gold-plated nickel alloy electroplating solution for electroplating, select the copper electrode as the cathode and the carbon plate as the anode. Adjust the temperature to a predetermined value at a current density of 1A / dm 2 ~5A / dm 2 Under plating.
[0032] The composition of the gold-plated nickel alloy electroplating solution is as follows:
[0033]
[0034] According to the electroplating step in the specific embodiment, the electroplating operation is performed using the cyanide-free gold-plated nickel alloy electroplating solution. Results The copper electrode was plated with gold-nickel alloy, and the weight percentage of gold was 95.83% (23K gold) through analysis and test.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com