A method for identifying aliphatic chain isomers α-ketoglutaric acid and 1,3-acetone dicarboxylic acid
A technology of isomers and acetone dicarboxylic acid, which is applied in the field of discrimination and identification, can solve the problems of rare identification methods and difficulty in distinguishing, and achieve the effects of easy operation and observation, high accuracy and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
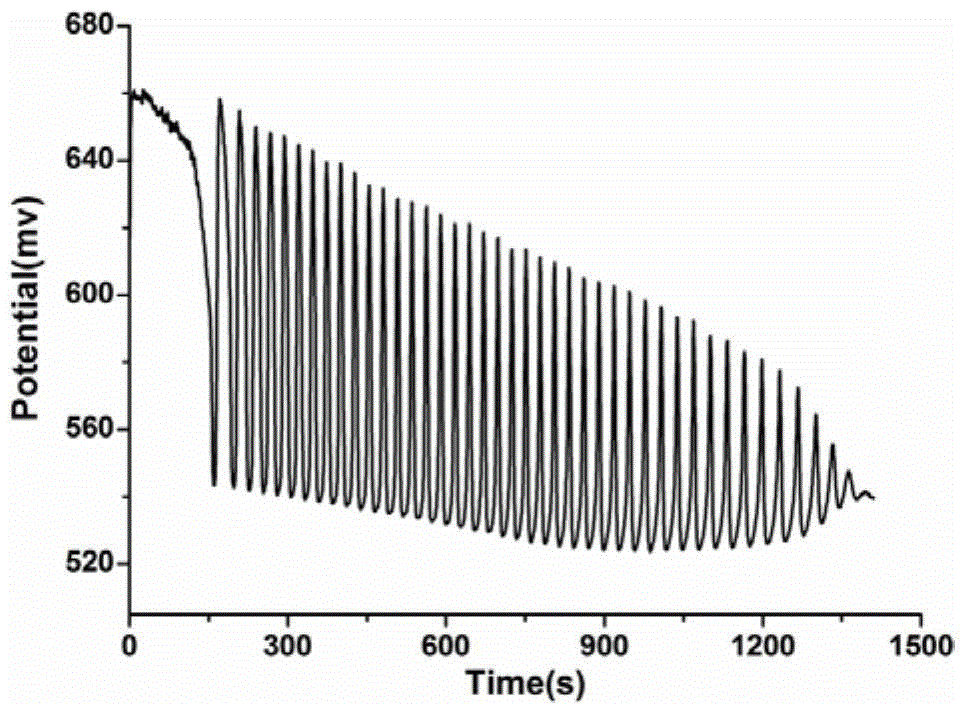
[0053] This example verifies the feasibility of the method for identifying aliphatic chain isomers α-ketoglutaric acid and 1,3-acetonedicarboxylic acid according to the following steps:
[0054] (1) Prepare the solution
[0055] First prepare the sulfuric acid of 0.025mol / L with 98% vitriol oil and distilled water as stock solution, then prepare respectively the potassium iodate solution of 0.14mol / L, the [NiL] of 0.0173mol / L with the sulfuric acid solution of 0.025mol / L ( ClO 4 ) 2 solution, 2mol / L malonic acid solution, 4mol / L hydrogen peroxide solution; add 14.5mL 0.025mol / L sulfuric acid solution, 6.5mL 0.14mol / L potassium iodate solution, 1.5mL 0.0173 mol / L[NiL](ClO 4 ) 2 solution, 3.5mL 2mol / L malonic acid solution and 14mL 4mol / L hydrogen peroxide solution to ensure that "H 2 SO 4 -KIO 3 -[NiL](ClO 4 ) 2 -MA-H 2 o 2 "The concentration of each component in the nonlinear chemical oscillation system is sulfuric acid 0.025mol / L, potassium iodate 0.02275mol / L, [Ni...
Embodiment 2
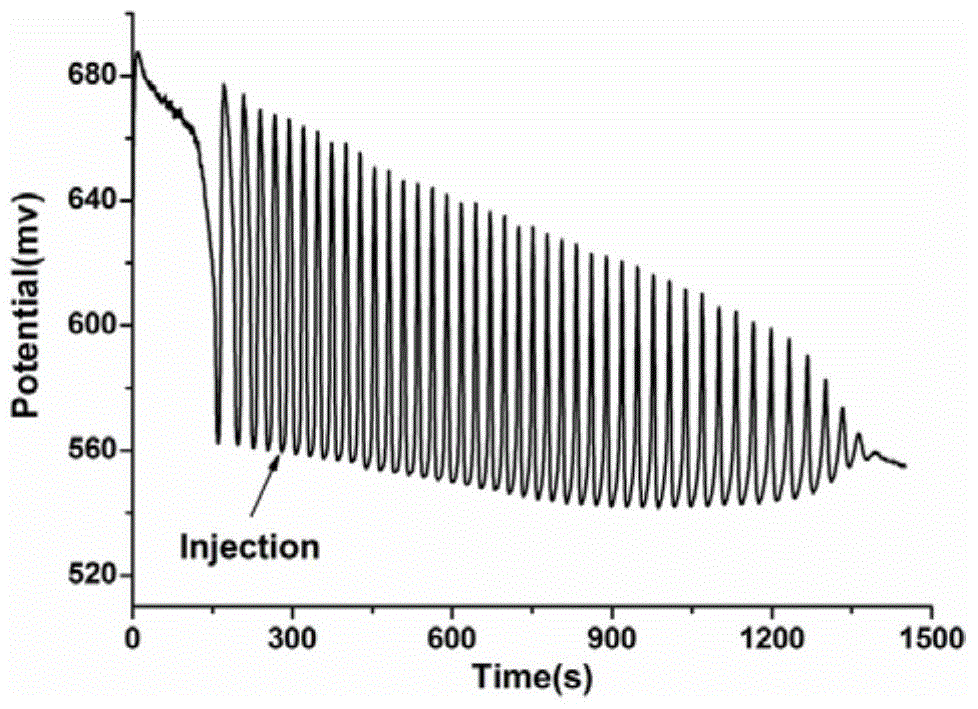
[0065] This example verifies the feasibility of the method for identifying aliphatic chain isomers α-ketoglutaric acid and 1,3-acetonedicarboxylic acid according to the following steps:
[0066] (1) Prepare the solution
[0067] First prepare 0.025mol / L sulfuric acid with 98% concentrated sulfuric acid as a stock solution, then prepare 0.14mol / L potassium iodate solution and 0.0173mol / L [NiL](ClO 4 ) 2 solution, 2mol / L malonic acid solution, 4mol / L hydrogen peroxide solution; add 13.5mL 0.025mol / L sulfuric acid solution, 6.5mL 0.14mol / L potassium iodate solution, 2mL 0.0173mol / L[NiL](ClO 4 ) 2 solution, 3.5mL 2mol / L malonic acid solution and 14.5mL 4mol / L hydrogen peroxide solution to ensure that "H 2 SO 4 -KIO 3 -[NiL](ClO 4 ) 2 -MA-H 2 o 2 "The concentration of each component in the nonlinear chemical oscillation system is sulfuric acid 0.025mol / L, potassium iodate 0.02275mol / L, [NiL](ClO 4 ) 2 8.65×10 -4 mol / L, malonic acid 0.175mol / L, hydrogen peroxide 1.45mo...
Embodiment 3
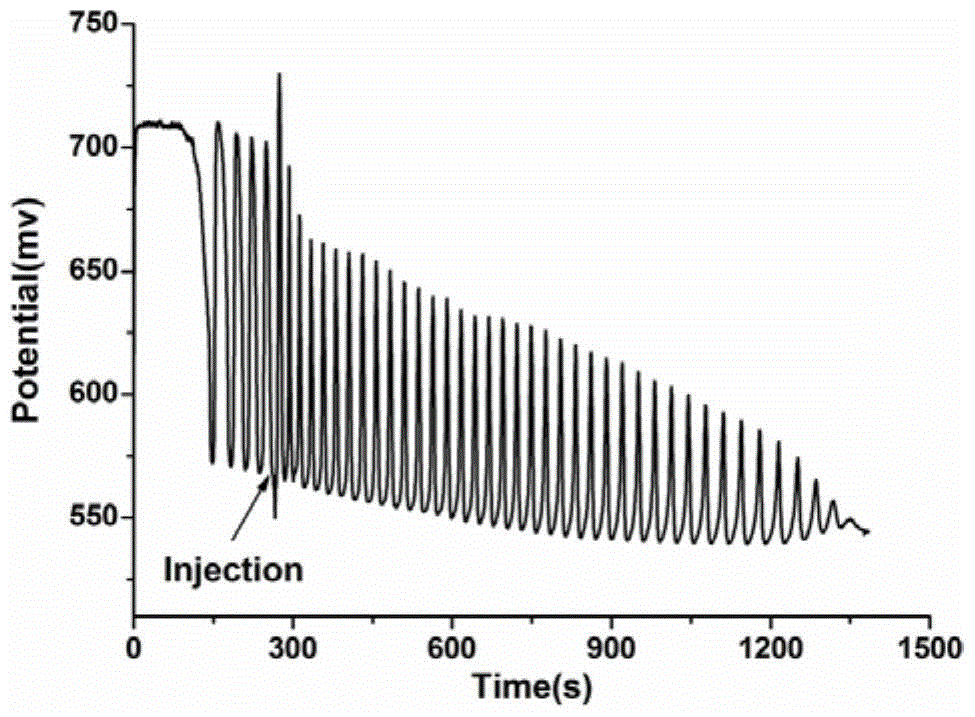
[0075] This example verifies the feasibility of the method for identifying aliphatic chain isomers α-ketoglutaric acid and 1,3-acetonedicarboxylic acid according to the following steps:
[0076] (1) Prepare the solution
[0077] First prepare 0.025mol / L sulfuric acid with 98% concentrated sulfuric acid as a stock solution, then prepare 0.14mol / L potassium iodate solution and 0.0173mol / L [NiL](ClO 4 ) 2 solution, 2mol / L malonic acid solution, 4mol / L hydrogen peroxide solution; add 15.5mL 0.025mol / L sulfuric acid solution, 6mL 0.14mol / L potassium iodate solution, 1.5mL 0.0173mol / L[NiL](ClO 4 ) 2 solution, 3mL 2mol / L malonic acid solution and 14mL 4mol / L hydrogen peroxide solution to ensure that "H 2 SO 4 -KIO 3 -[NiL](ClO 4 ) 2 -MA-H 2 o 2 "The concentration of each component in the nonlinear chemical oscillation system is sulfuric acid 0.025mol / L, potassium iodate 0.021mol / L, [NiL](ClO 4 ) 2 6.4875×10 - 4 mol / L, malonic acid 0.15mol / L, hydrogen peroxide 1.4mol / L;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com