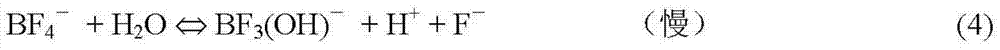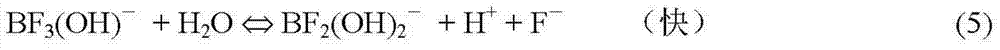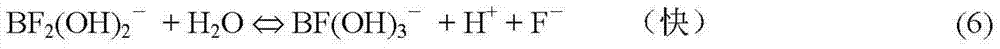Method for analyzing fluorine ion concentration under radioactive condition
An analytical method and radioactive technology, applied in the field of analysis of fluoride ion concentration under radioactive conditions, can solve the problems of unreported, slow dissolution rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] (1) Preparation of total ionic strength adjustment buffer solution (TISAB for short): prepare a mixed solution of sulfosalicylic acid and trisodium citrate, and adjust the pH to 9 with ammonia water;
[0072] (2) Preparation of F ion standard solution:
[0073] Prepare F ion standard solution A (concentration is 0.5mol / L): Accurately weigh 2.0995g of reference NaF (pre-dried at 105°C to 110°C for more than 2h), dissolve it in deionized water and set to volume in a 100mL volumetric flask;
[0074] Prepare F ion standard solution B (concentration is 0.1mol / L): accurately pipette 20mL F ion standard solution A into a 100mL volumetric flask, and dilute to volume with deionized water;
[0075] Prepare F ion standard solution C (concentration is 0.01mol / L): accurately pipette 10mL F ion standard solution B into a 100mL volumetric flask, and dilute to volume with deionized water;
[0076] (3) Prepare aluminum nitrate solution, uranyl nitrate and nitric acid solution:
[0077...
Embodiment 2
[0086] On the basis of Example 1, for HBF 4 The dissociation of fluoride ions in the solution was analyzed.
[0087] Prepare total ionic strength adjustment buffer solution (TISAB for short): prepare mixed solution, wherein the concentration of sulfosalicylic acid is 0.5mol / L, the concentration of trisodium citrate is 0.3mol / L, and adjust pH8.5 with ammonia water;
[0088] Take 0.5mL (V 样品 ) with a concentration of 0.01mol / L of HBF 4 solution in V x =50mL volumetric flask, then add 25mLTISAB, 0.32mL nitric acid solution with a concentration of 2mol / L, 1mL aluminum nitrate solution with a concentration of 1mol / L, 1mL uranyl nitrate solution with a concentration of 0.01mol / L, and then deionized The water is fixed to 50mL; then it is completely transferred to the measuring cup, and the solution potential E is measured with an ion meter (ion meter: equipped with a fluoride ion selective electrode, a glass electrode and a reference electrode, accurate to 0.1mV) 1 (230.83mV); Ad...
Embodiment 3
[0091] Prepare total ionic strength adjustment buffer solution (TISAB for short): prepare mixed solution, wherein the concentration of sulfosalicylic acid is 0.3mol / L, the concentration of trisodium citrate is 0.3mol / L, and adjust pH8 with ammonia water;
[0092] Get the F ion standard solution B 0.5mL (V 样品 ) at V x=50mL volumetric flask, then add 25mL TISAB, 0.32mL concentration is 2mol / L nitric acid solution, 0.25mL concentration is 1mol / L aluminum nitrate solution, then add 1mL concentration is 0.005mol / L uranyl nitrate solution ( 0.01mol / L uranyl nitrate solution diluted 2 times) and then dilute to 50mL with deionized water; electrode, accurate to 0.1mV) to measure the solution potential E 1 (159.38mV); Add the prepared F ion standard solution (concentration is Cs) B 0.5mL (V 1 ), the solution potential E was measured with an ion meter 2 (142.67mV); Continue to add the prepared F ion standard solution (concentration is Cs) A 1mL (V 2 ), the solution potential E was m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com