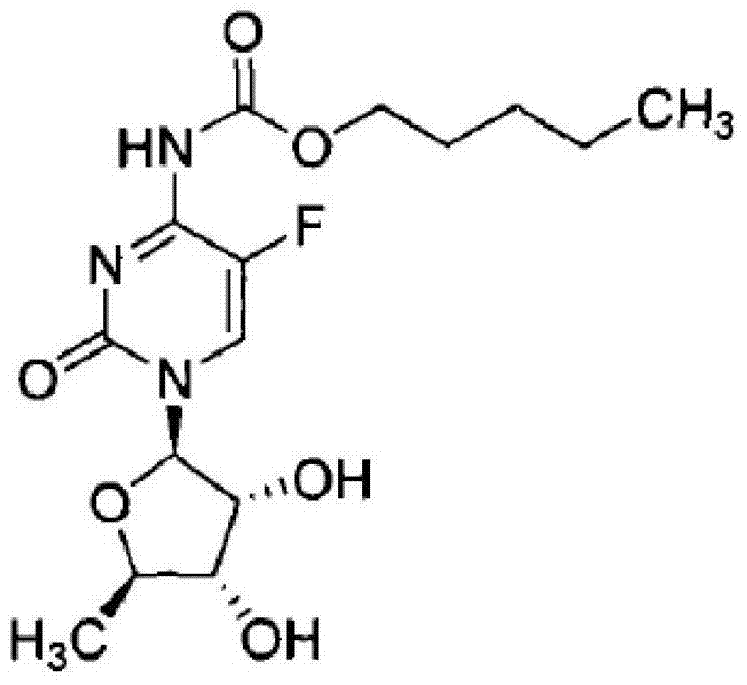
Synthetic method of capecitabine
A synthetic method and capecitabine technology, applied in the field of drug preparation, can solve problems such as increased pollution, health hazards of workers, and increased costs, and achieve the effects of ensuring health, facilitating industrial production, and increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Under ice bath, control the temperature at 5°C, slowly drop n-amyl chloroformate (21.5mmol) into K 2 CO 3 (21.7mmol), dimethylaminopyridine (2.46mmol) and 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (14.5mmol) in dichloromethane (30mL) , keep warm for 45 minutes. After filtration, the filtrate was washed successively with 0.2M hydrochloric acid (10 mL), water (10 mL) and saturated brine (10 mL), dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a white solid (6.1 g, yield 90.6%).
[0020] Add N-pentyloxycarbonyl-2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (5 g) and methanol (40 mL) into a 100 mL three-necked flask. After cooling to 0°C, 10 mL of 2M sodium hydroxide was added dropwise, and the temperature was maintained at 5 to 15°C. After reacting for 1 hour, 2M hydrochloric acid was added dropwise to adjust the pH value to about 6. After three extractions with dichloromethane (150 mL), the combined organic phases were ...
Embodiment 2
[0022] Under ice bath, the temperature was controlled to be 15°C, and n-amyl chloroformate (20.4 mmol) was slowly dropped into Na 2 CO 3 (20.3mmol), dimethylaminopyridine (2.67mmol) and 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (14.5mmol) in chlorothane (30mL) solution, Insulation reaction 45min. After filtration, the filtrate was washed successively with 0.2M hydrochloric acid (10 mL), water (10 mL) and saturated brine (10 mL), dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a white solid (5.9 g, yield 87.6%).
[0023] Add N-pentyloxycarbonyl-2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (5 g) and methanol (40 mL) into a 100 mL three-necked flask. After cooling to 0°C, 11 mL of 2M potassium hydroxide was added dropwise, and the temperature was maintained at 5 to 15°C. After reacting for 1 hour, 2M hydrochloric acid was added dropwise to adjust the pH to about 6. After three extractions with dichloromethane (150 mL), the com...
Embodiment 3
[0025] Under ice bath, the temperature was controlled to be 1°C, and n-amyl chloroformate (23.1 mmol) was slowly dropped into K 2 CO 3 (22.6mmol), dimethylaminopyridine (2.65mmol) and 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (14.5mmol) in toluene (30mL) solution, incubated React for 45 minutes. After filtration, the filtrate was washed successively with 0.2M hydrochloric acid (10 mL), water (10 mL) and saturated brine (10 mL), dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a white solid (5.7 g, yield 84.7%).
[0026] Add N-pentyloxycarbonyl-2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (5 g) and methanol (40 mL) into a 100 mL three-necked flask. After cooling to 0°C, 12 mL of 2M sodium methoxide was added dropwise, and the temperature was maintained at 5 to 15°C. After reacting for 1 hour, 2M hydrochloric acid was added dropwise to adjust the pH value to about 6. After three extractions with dichloromethane (150 mL), the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com