Method for determining enantiomer impurity in alogliptin crude drug and preparation by virtue of HPLC
A technology of enantiomers and raw materials, applied in the field of pharmaceutical analysis, can solve problems such as ineffectiveness, isomer impurity detection methods that have not yet been patented and reported in literature, and harmful
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Instrument: AgiLent1100 high performance liquid chromatography, 1100 UV detector;
[0105] Chromatographic column: CHIRALPAK AS-H (150×4.6mm, 5μm);
[0106] Mobile phase A: n-hexane (containing 0.3% diethylamine v / v);
[0107] Mobile phase B: ethanol (containing 0.3% diethylamine v / v);
[0108] See the table below for gradient elution;
[0109] time (minutes)
Mobile Phase A(%)
Mobile phase B(%)
0
75
25
3
75
25
20
25
75
25
25
75
26
75
25
32
75
25
[0110] Flow rate: 0.4mL / min
[0111] Detection wavelength: 278nm
[0112] Column temperature: 25°C
[0113] Injection volume: 10μL
[0114] Thinner: ethanol
[0115] experiment procedure:
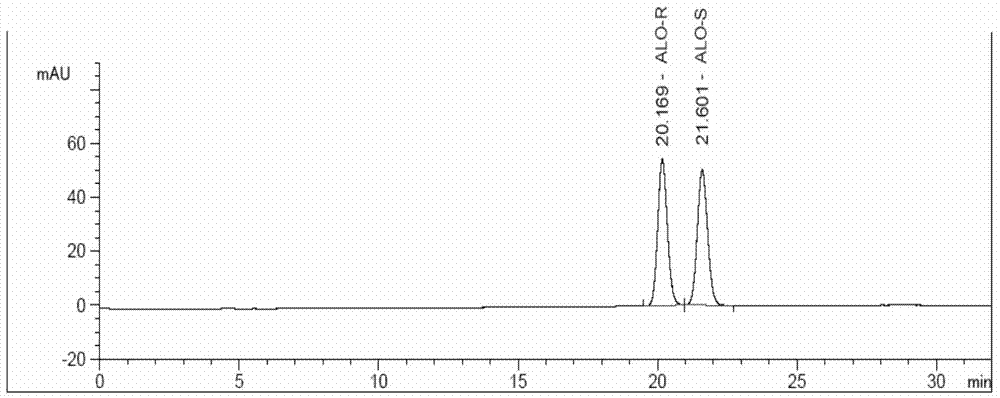
[0116] Separation test solution: Accurately weigh 5.12mg of alogliptin benzoate racemate (a mixture of alogliptin benzoate R type and S type about 1:1) into a 100mL volumetric flask, add an appropriate amount of ethanol,...
Embodiment 2
[0127] Instrument: AgiLent1100 high performance liquid chromatography, 1100 UV detector;
[0128] Chromatographic column: CHIRALPAK AS-H (150×4.6mm, 5μm);
[0129] Mobile phase A: n-hexane (containing 0.2% diethylamine v / v);
[0130] Mobile phase B: ethanol (containing 0.2% diethylamine v / v);
[0131] See the table below for gradient elution;
[0132] time (minutes)
Mobile Phase A(%)
Mobile phase B(%)
0
75
25
3
75
25
20
25
75
25
25
75
26
75
25
32
75
25
[0133] Flow rate: 0.4mL / min
[0134] Detection wavelength: 278nm
[0135] Column temperature: 25°C
[0136] Injection volume: 10μL
[0137] Thinner: ethanol
[0138] experiment procedure:
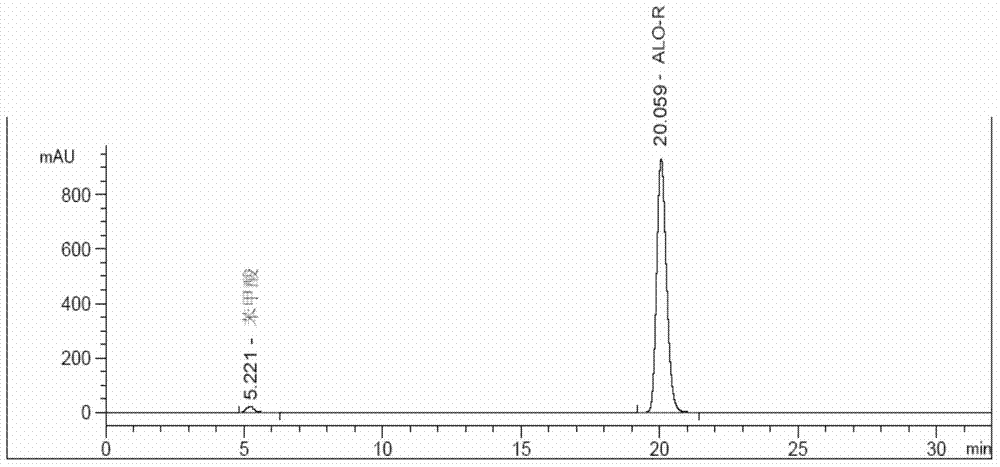
[0139] Separation test solution: Accurately weigh 5.23 mg of alogliptin racemate (about 1:1 mixture of alogliptin R type and S type) into a 10mL volumetric flask, add an appropriate amount of ethanol, dissolve it by ultr...
Embodiment 3
[0143] Instrument: AgiLent1100 high performance liquid chromatography, 1100 UV detector;
[0144] Chromatographic column: CHIRALPAK AS-H (150×4.6mm, 5μm);
[0145] Mobile phase A: n-hexane (containing 0.4% diethylamine v / v);
[0146] Mobile phase B: ethanol (containing 0.4% diethylamine v / v);
[0147] See the table below for gradient elution;
[0148] time (minutes)
Mobile Phase A(%)
Mobile phase B(%)
0
75
25
3
75
25
20
25
75
25
25
75
26
75
25
32
75
25
[0149] Flow rate: 0.4mL / min
[0150] Detection wavelength: 278nm
[0151] Column temperature: 25°C
[0152] Injection volume: 10μL
[0153] Thinner: ethanol
[0154] experiment procedure:
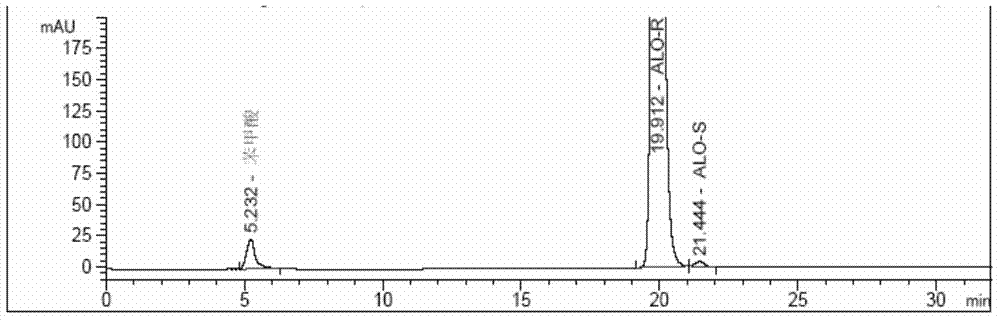
[0155] Separation test solution: Accurately weigh 5.45 mg of alogliptin racemate (about 1:1 mixture of alogliptin R type and S type) into a 10mL volumetric flask, add an appropriate amount of ethanol, dissolve it by ultr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com