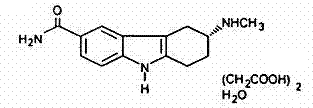
Frovatriptan succinate tablet and preparation method thereof
A technology for furotriptan tablets and furotriptan, which is applied in the field of medicine, can solve problems such as failure to provide preparations, influence of pharmaceutical agents, and many excipients, and achieves a simple and easy process route, stable quality, and reasonable method selection. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
Embodiment 2
[0025]
Embodiment 3
[0027]
[0028] The preparation technology of embodiment 1-3 is as follows:
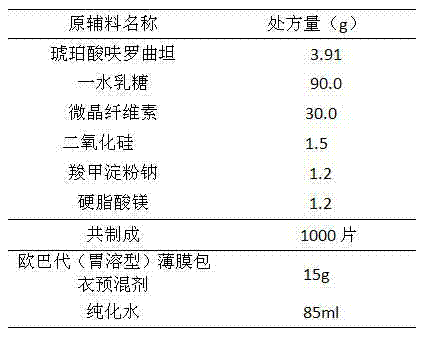
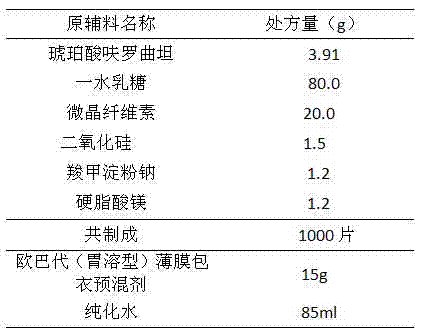
[0029] a. pass the bulk drug through an 80-mesh sieve for subsequent use; weigh the microcrystalline cellulose, lactose monohydrate, silicon dioxide, and sodium starch glycolate of the prescribed amount for subsequent use;
[0030] b. Mix the main ingredient with microcrystalline cellulose, silicon dioxide, sodium starch glycolate; mix with lactose monohydrate;
[0031] c. Add the magnesium stearate of prescribed quantity and mix and then compress into tablets;
[0032] d. Take Opadry dry powder and add it into purified water under stirring, stir until dispersed, and filter until it dissolves evenly; coat with film;
[0033] e. Finished product inspection and packaging.
[0034] According to the Chinese Pharmacopoeia 2010 edition, each embodiment was tested and compared, and the comparison results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com