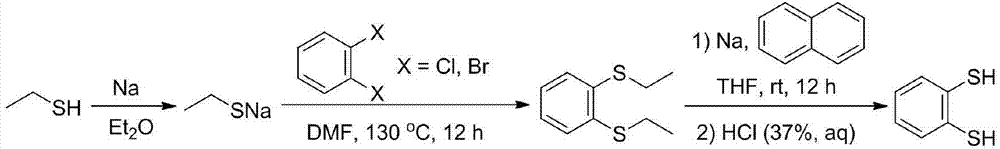
Method for preparing benzene-1,2-dithiol
A technology of ortho-dithiol and ortho-dithiol, which is applied in the field of medicinal chemistry, can solve the problems of complex operation, research, and high cost of raw materials, and achieve the effects of mild reaction conditions, wide application prospects, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
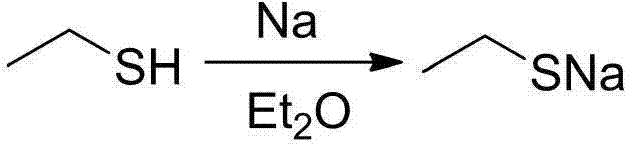
[0022] Under the protection of argon, 40 mL of freshly dried diethyl ether and 1.15 g of metallic sodium (50 mmol) were added to a 100 mL reaction flask with a branched opening. While stirring, 4.07 mL of ethanethiol (55 mmol) was added dropwise to the reaction solution, and the reaction was stirred at room temperature for 4 hours until the metallic sodium completely disappeared and a large amount of white turbidity was generated. Then ether and a small part of excess ethanethiol were removed under reduced pressure, and the obtained white powder was sodium ethanethiolate (yield: 3.88 g, yield: 92.4%), which was directly used in the next reaction.
example 2
[0024] Under the protection of argon, 40 mL of freshly dried diethyl ether and 1.15 g of metallic sodium (50 mmol) were added to a 100 mL reaction flask with a branched opening. While stirring, 7.4 mL of ethanethiol (100 mmol) was added dropwise to the reaction solution, and the reaction was stirred at room temperature for 4 hours until the metallic sodium completely disappeared and a large amount of white turbidity was generated. Then ether and a small part of excess ethanethiol were removed under reduced pressure, and the obtained white powder was sodium ethanethiolate (yield: 4.07 g, yield: 96.9%), which was directly used in the next reaction.
example 3
[0026] Under the protection of argon, 40 mL of freshly dried diethyl ether and 1.15 g of metallic sodium (50 mmol) were added to a 100 mL reaction flask with a branched opening. 11.1 mL of ethanethiol (150 mmol) was added dropwise to the reaction solution under stirring, and the reaction was stirred at room temperature for 3 hours until the metallic sodium completely disappeared and a large amount of white turbidity was generated. Then ether and a small part of excess ethanethiol were removed under reduced pressure, and the obtained white powder was sodium ethanethiolate (yield: 4.18 g, yield: 99.5%), which was directly used in the next reaction.
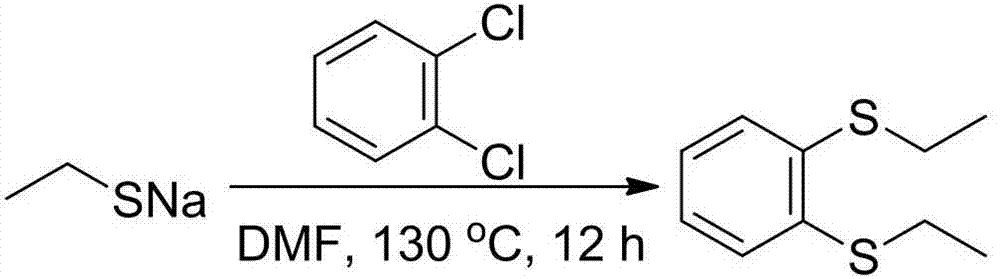
[0027] (2) The following will combine image 3 and Figure 4 Describe in detail the steps of preparing 1,2-diethylthiobenzene from the sodium ethanethiolate prepared in step (1).
[0028] 1. Prepare 1,2-diethylthiobenzene with o-dichlorobenzene as raw material (see image 3 )
[0029] Example 1:
[0030] Weigh 1.68g (20mmol) of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com