Fludarabine phosphate freeze-dried powder injection
A technology of fludarabine phosphate and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations, can solve the problems of difficulty in ensuring the moisture content of the finished product and long use time, and achieve the effect of safe effect, stable quality and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Fludarabine Phosphate 5g
[0025] Water for injection 100ml
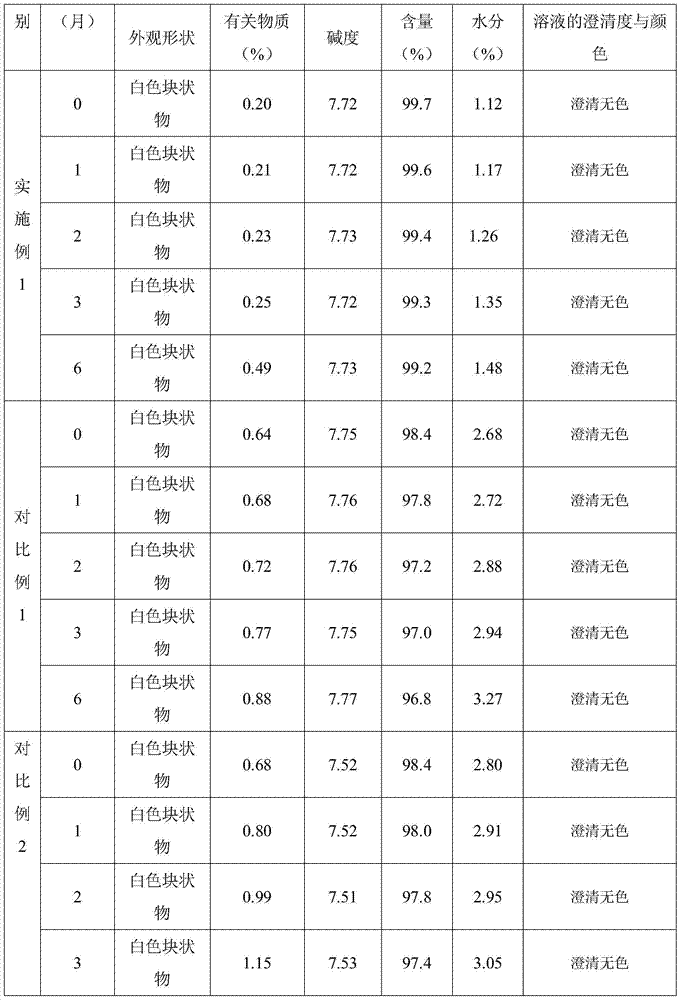
[0026] Weigh 5g of the 75-100μm fludarabine phosphate powder obtained by micronization, add 100ml of water for injection, use sodium hydroxide to adjust the pH value to 7.2-8.2, stir to dissolve completely, and fill each 1ml into a 10ml vial middle. The plate layer of the lyophilizer was cooled to -45°C, and after filling, the vials were put into the lyophilizer and kept warm for 2 hours. Raise the temperature of the plate layer to -8°C, keep it warm for 2 hours, then cool the plate layer down to -45°C, and keep it warm for 3 hours. Heat the plate layer to 20°C within 8 to 12 hours; keep warm at 35°C for 8 hours, fill it with a nitrogen pressure plug, take out the crimped cap and pack it.
Embodiment 2
[0028] Fludarabine Phosphate 5g
[0029] Water for injection 100ml
[0030] Weigh 5g of the 75-100μm fludarabine phosphate powder obtained by micronization, add 100ml of water for injection, use sodium hydroxide to adjust the pH value to 7.2-8.2, stir to dissolve completely, and fill each 1ml into a 10ml vial middle. The plate layer of the lyophilizer was cooled to -45°C, and after filling, the vials were put into the lyophilizer and kept warm for 4 hours. Raise the temperature of the plate layer to -8°C, keep the temperature for 2 hours, then cool down the plate layer to -40°C, and keep the heat preservation for 3 hours. Heat the plate layer to 15°C within 8 to 12 hours; keep warm at 40°C for 10 hours, fill in the nitrogen pressure plug, take out the crimped cap and pack it.
Embodiment 3
[0032] Fludarabine Phosphate 5g
[0033] Water for injection 100ml
[0034] Weigh 5g of the 75-100μm fludarabine phosphate powder obtained by micronization, add 100ml of water for injection, use sodium hydroxide to adjust the pH value to 7.2-8.2, stir to dissolve completely, and fill each 1ml into a 10ml vial middle. The plate layer of the lyophilizer is cooled to -50°C, and after filling, the vials are put into the lyophilizer and kept warm for 1 hour. Raise the temperature of the plate layer to -8°C, keep the temperature for 2 hours, then cool down the plate layer to -40°C, and keep the heat preservation for 3 hours. Heat the plate layer to 25°C within 8 to 12 hours; keep warm at 30°C for 10 hours, fill in the nitrogen pressure plug, take out the crimped cap and pack it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com