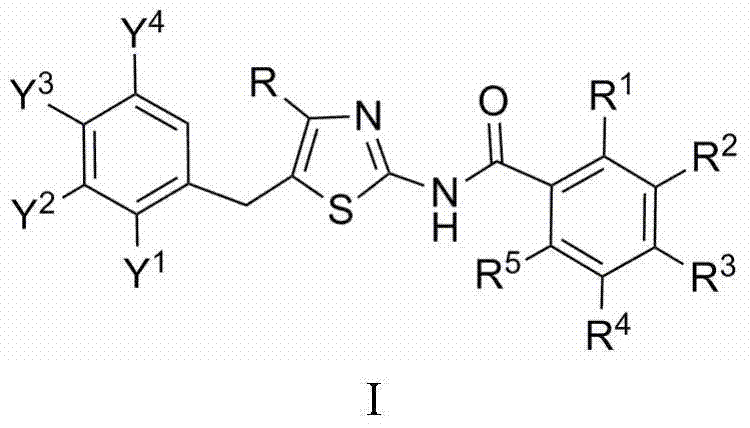
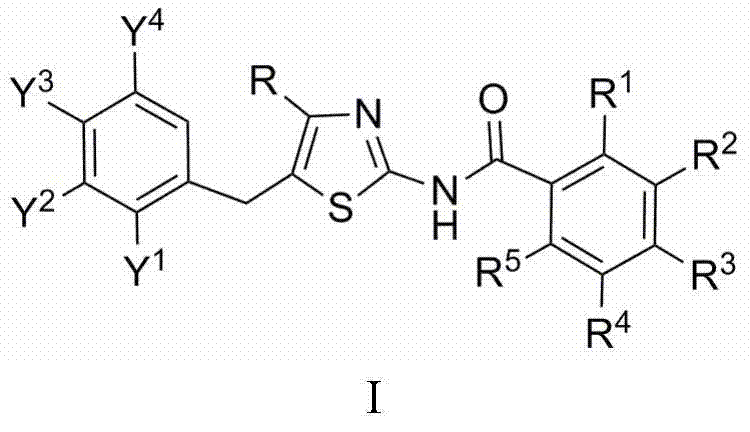
N-(5-benzyl thiazole-2-yl)benzamide, and pharmaceutical applications thereof
A technology of benzylthiazole and benzamide, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Preparation of N-[4-tert-butyl-5-(2,4-dichlorobenzyl)thiazol-2-yl]-2,6-difluorobenzamide
[0014] 1.5mmol 4-tert-butyl-5-(2,4-dichlorobenzyl)thiazol-2-amine, 1.6mmol 2,6-difluorobenzoic acid and 40mL dichloromethane, stirred at room temperature, added 0.15mmol 4-dimethylamino Pyridine, stirred for 0.5h, added 1.6mmol N,N'-dicyclohexylcarbodiimide, and TLC monitored the reaction. After the reaction was completed, the column was passed to obtain N-[4-tert-butyl-5-(2,4-dichlorobenzyl)thiazol-2-yl]-2,6-difluorobenzamide with a yield of 78.1%. m.p.133~135℃. 1 H NMR (CDCl 3 , 400MHz), δ: 1.32 (s, 9H, 3×CH 3 ), 4.29 (s, 2H, CH 2 ), 7.00~7.51 (m, 6H, 2×C 6 h 3 ), 9.40 (br, 1H, NHCO).
Embodiment 2
[0016] Preparation of N-[4-tert-butyl-5-(2,4-dichlorobenzyl)thiazol-2-yl]-2-chlorobenzamide
[0017] 1.5mmol 4-tert-butyl-5-(2,4-dichlorobenzyl)thiazol-2-amine, 1.6mmol 2-chlorobenzoic acid and 40mL dichloromethane, stirred at room temperature, added 0.15mmol 4-dimethylaminopyridine, stirred 0.5h, 1.6mmol N,N'-dicyclohexylcarbodiimide was added, and the reaction was monitored by TLC. After the reaction was completed, the column was passed to obtain N-[4-tert-butyl-5-(2,4-dichlorobenzyl)thiazol-2-yl]-2-chlorobenzamide with a yield of 78.3%, m.p.148~ 150°C. 1 H NMR (CDCl 3 , 400MHz), δ: 1.34 (s, 9H, 3×CH 3 ), 4.30 (s, 2H, CH 2 ), 7.08 (d, J=8.4Hz, 1H, C 6h 3 6-H), 7.18 (dd, J=8.4Hz, J=2.0Hz, 1H, C 6 h 3 5-H), 7.39~7.81 (m, 5H, ClC 6 h 3 3-H,C 6 h 4 ), 9.50 (br, 1H, NHCO).
Embodiment 3
[0019] Preparation of N-[4-tert-butyl-5-(2,4-dichlorobenzyl)thiazol-2-yl]-4-chlorobenzamide
[0020] 1.5mmol 4-tert-butyl-5-(2,4-dichlorobenzyl)thiazol-2-amine, 1.6mmol 4-chlorobenzoic acid and 40mL dichloromethane, stirred at room temperature, added 0.15mmol 4-dimethylaminopyridine, stirred 0.5h, 1.6mmol N,N'-dicyclohexylcarbodiimide was added, and the reaction was monitored by TLC. After the reaction was completed, the column was passed to obtain N-[4-tert-butyl-5-(2,4-dichlorobenzyl)thiazol-2-yl]-4-chlorobenzamide, yield 74.5%, m.p.137~ 140°C. 1 H NMR (CDCl 3 , 400MHz), δ: 1.36 (s, 9H, 3×CH 3 ), 4.29 (s, 2H, CH 2 ), 7.06 (d, J=8.4Hz, 1H, C 6 h 3 6-H), 7.18 (dd, J=8.4Hz, J=2.4Hz, 1H, C 6 h 3 5-H), 7.41 (d, J=2.4Hz, 1H, C 6 h 3 3-H), 7.49 (d, J=8.8Hz, 2H, C 6 h 4 3,5-H), 7.89 (d,, J=8.8Hz, 2H, C 6 h 4 2,6-H), 9.57 (br,1H, NHCO).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com