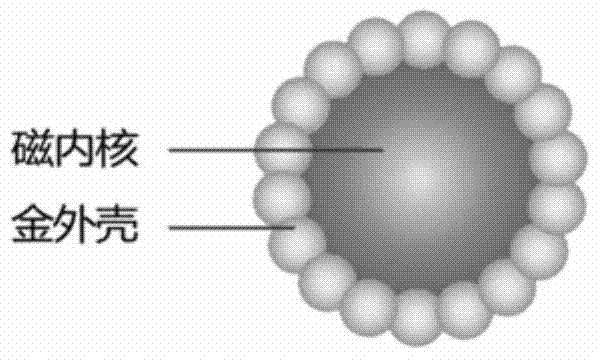
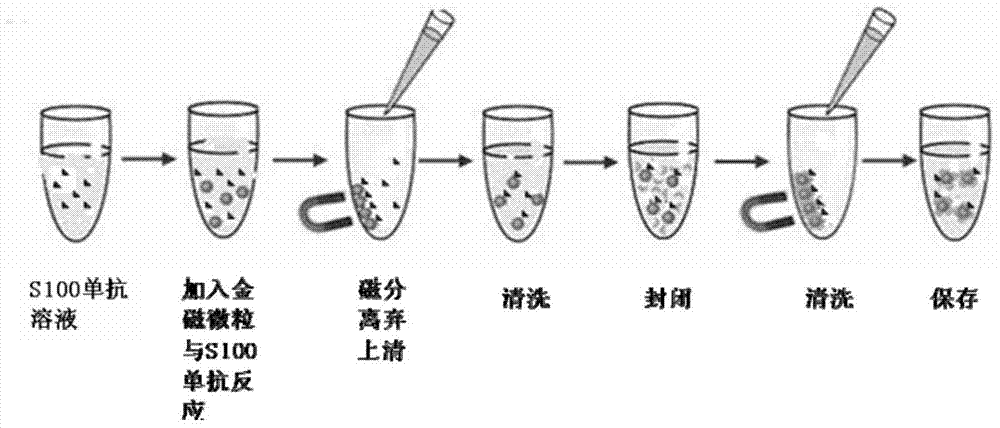
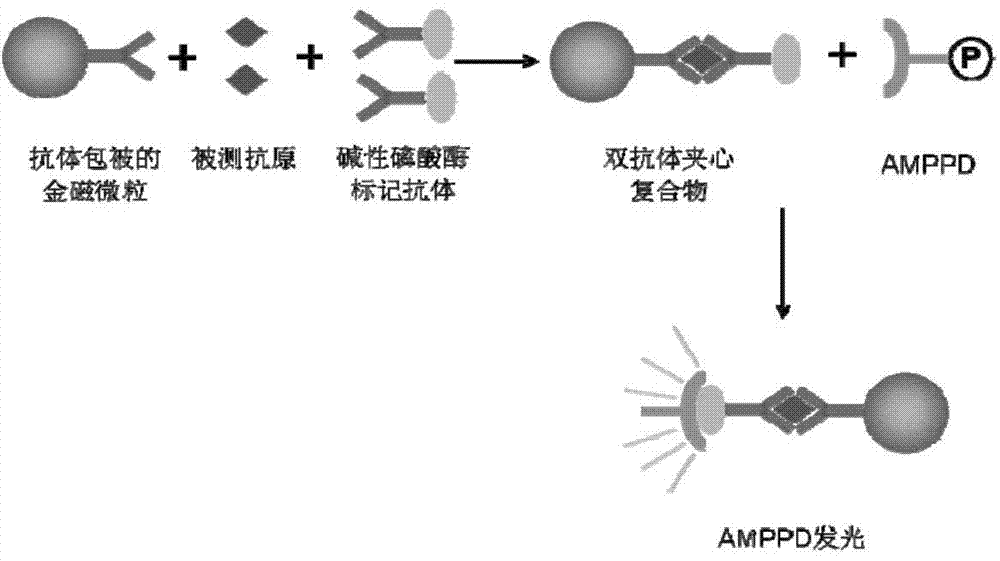
Chemiluminescence immunoassay method for detecting S100 based on gold magnetic particles
A technology of chemiluminescent immunity and gold magnetic particles, which is applied in biological testing, material inspection products, etc., can solve the problems of antigen or antibody inactivation, complex coupling process, and restrictions on popularization and application, achieving high precision and good specificity , Operational safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Method for Quantitative Detection of S100 in Human Serum by Chemiluminescence Immunology Based on Gold Magnetic Particles
[0080] (1) Coated
[0081] (1.1) Pretreatment: Take 100 μl of 10 mg / ml gold magnetic particles, wash them twice with 100 μl of pH 7.4, 0.02M Tris-HCl equilibrium buffer to balance the pH of the magnetic particles.
[0082] (1.2) Coupling: Dissolve 160 μg S100 antibody in 200 μl pH7.4, 0.02M Tris-HCl coupling buffer, mix well and add to pretreated gold magnetic particles, shake at 37°C and 180rpm React in bed for 35min. After the reaction is complete, take it out, magnetically separate it for 2 minutes, and discard the supernatant.
[0083] (1.3) Washing: add 200 μl pH7.4, 0.02M Tris-HCl washing buffer containing 0.05% Tween-20, perform magnetic separation for 2 min, and discard the supernatant.
[0084] (2) Sealing: add 1ml containing 5% bovine serum albumin, 2.5% skimmed milk powder and 3% fetal bovine serum pH8.0, in the 0.01M PB bu...
Embodiment 2
[0089] Example 2: Method for Quantitative Detection of S100 in Human Serum by Chemiluminescence Immunology Based on Gold Magnetic Particles
[0090] (1) Coated
[0091] (1.1) Pretreatment: Take 100 μl of 10 mg / ml gold magnetic particles, wash them twice with 100 μl of pH 7.6, 0.02M Tris-HCl equilibrium buffer to balance the pH of the magnetic particles.
[0092] (1.2) Coupling: Dissolve 150 μg S100 antibody in 200 μl pH7.6, 0.02M Tris-HCl coupling buffer, mix well and add to pretreated gold magnetic particles, shake at 35°C, 200rpm The bed reacted for 38min. After the reaction is complete, take it out, magnetically separate it for 2 minutes, and discard the supernatant.
[0093] (1.3) Washing: Add 200 μl pH7.6, 0.02M Tris-HCl washing buffer containing 0.07% Tween-20, magnetically separate for 2 min, and discard the supernatant.
[0094] (2) Sealing: add 1ml containing 5% bovine serum albumin, 2.5% skimmed milk powder and 3% fetal bovine serum pH8.0, in the 0.01M PB buffer s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com