Method for preparing lithium salt through recycling lithium fluoride-containing waste material material
A technology containing lithium fluoride and lithium fluoride, applied in the direction of lithium halide, etc., can solve problems such as environmental pollution and waste of lithium resources, and achieve the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
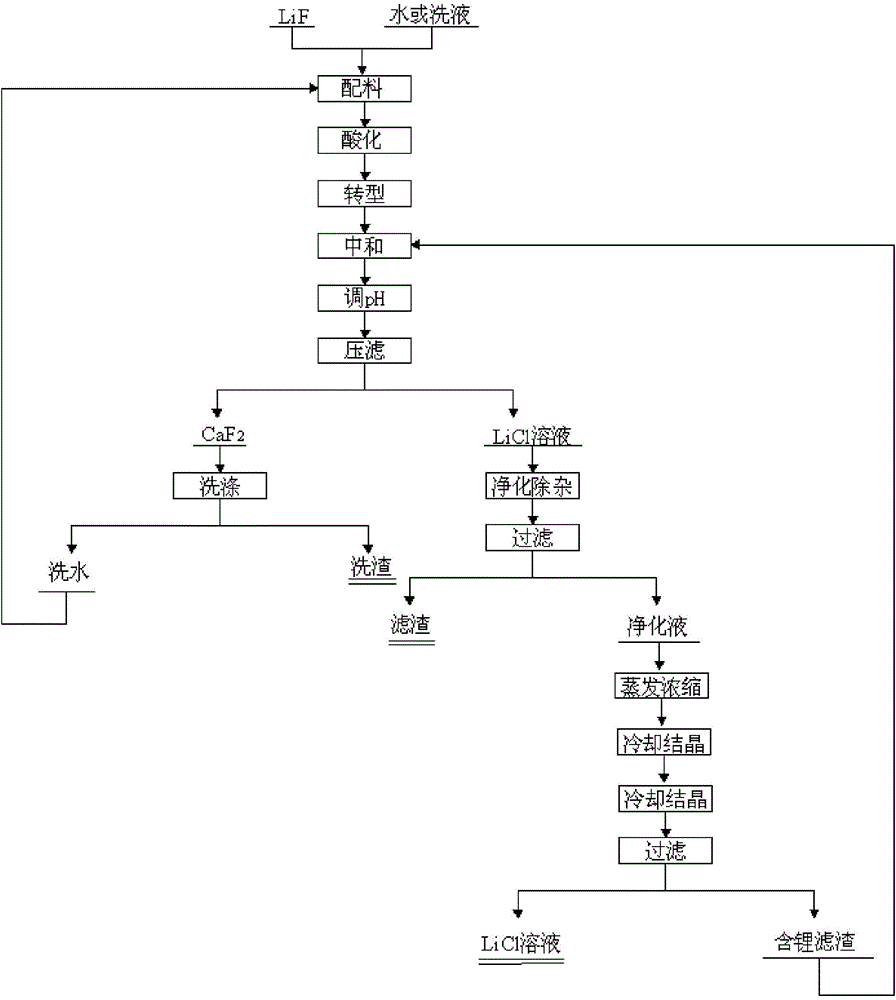
Image
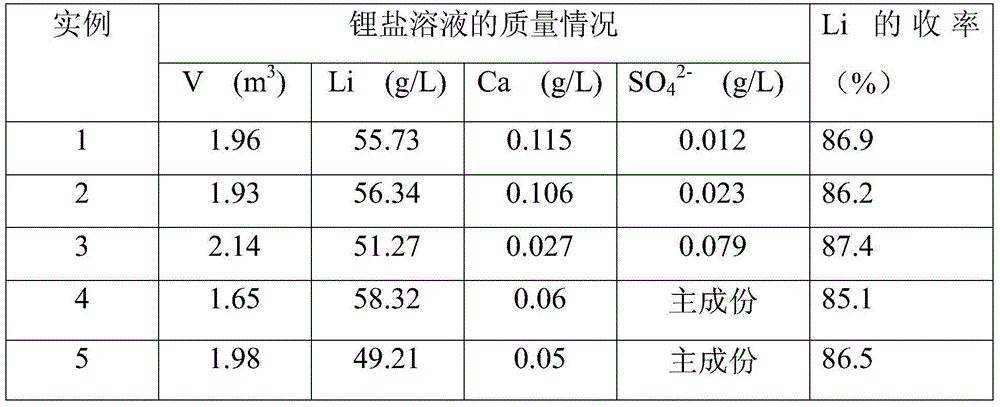
Examples
Embodiment 1
[0046] (1) add 420kg lithium fluoride waste material and 4m 3 Pure water, stirred evenly to form a slurry, heated up to 20°C; (2) adding 280kg of HCl solution with a concentration of 31% to the slurry, adjusting the pH of the slurry to 0, and stirring for 10 minutes of acidification reaction; (3) to the reaction Add 35% CaCl to the kettle 2 The solution was 2440kg, stirred for 30 minutes, and the CaCl was added within 10 minutes. 2 solution, stirring and reacting for 1h; (4) adding CaCO to the mixed solution obtained in step (3) 3Adjust the pH of the solution to be 6; (5) add NaOH with a concentration of 32% to the mixed solution to adjust the pH of the solution to 10; (6) filter the mixed solution and wash the filter residue 2 times; (7) according to step (6) Calcium in the filtrate that obtains, sulfate ion content adds the Na of calculated amount according to excess 1%. 2 CO 3 and BaCl 2 .8H 2 O, obtained after filtration about 5.5m 3 Purification solution; (8) the p...
Embodiment 2
[0048] (1) Add 420kg lithium fluoride waste and 2.1m 3 The lotion is evenly stirred to form a slurry, and the temperature is raised to 95° C.; (2) adding 280 kg of HCl solution with a concentration of 31% to the slurry, adjusting the pH of the slurry to 0, stirring and acidifying for 60 minutes; (3) going to the reaction Add 10% CaCl to the kettle 2 The solution was 7840kg, stirred for 60 minutes, and the CaCl was added within 60 minutes. 2 solution; (4) add CaCO to the mixed solution that step (3) obtains 3 The pH of the adjusting solution is 7; (5) adding concentration to the mixed solution is 32% KOH, adjusting the pH of the solution to 12; (6) filtering the mixed solution, washing the filter residue 4 times; (7) according to step (6) In the filtrate obtained by adding Na in excess of 2% 2 CO 3 and BaCl 2 .8H 2 O, 8.5m obtained after filtration 3 Purification solution; (8) the purification solution is concentrated to Li + The content is 55g / L, and the lithium chlori...
Embodiment 3
[0050] (1) add 420kg lithium fluoride waste material and 3m 3 The lotion is stirred evenly to form a slurry, and the temperature is raised to 60° C.; (2) adding 150 kg of HNO with a concentration of 65% to the slurry 3 solution, adjust the pH of the slurry to be 0, stir and acidify for 35min; (3) add a concentration of 35% Ca(NO 3 ) 2 Solution 4250kg, stirring reaction 45min, control to add Ca(NO 3 ) 2 solution; (4) add Ca(OH) to the mixed solution that step (3) obtains 2 The pH of the adjustment solution is 6.5; (5) adding concentration to the mixed solution is 32% liquid caustic soda, and adjusts the pH of the solution to 12; (6) the mixed solution is filtered, and the filter residue is washed 3 times; (7) according to calcium, sulfuric acid Radical ion content adds Na in the filtrate that obtains in step (6) by excessive 3%. 2 CO 3 and BaCl 2。 8H 2 O, obtained after filtration about 6.5m 3 Purification solution; (8) the purification solution is concentrated to Li ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com