Method for preparing 2, 6-difluorobenzonitrile
A technology of difluorobenzonitrile and dichlorobenzonitrile, which is applied in the field of pesticide preparation, can solve problems such as low yield, cumbersome process, complicated post-treatment, etc., achieve high melting point, increase reaction yield, and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
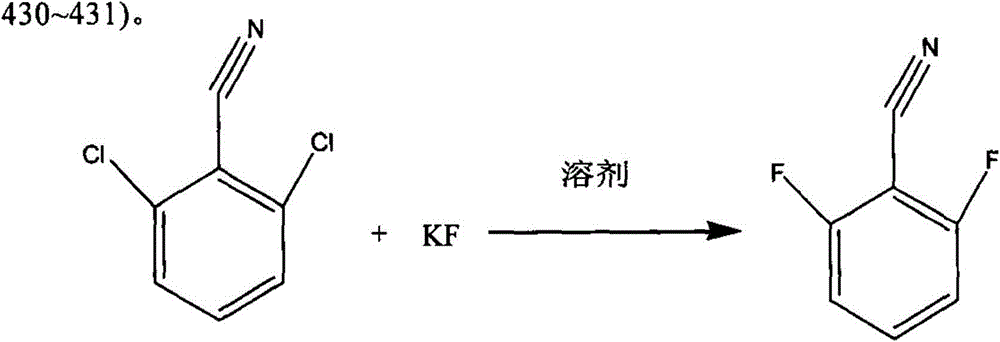
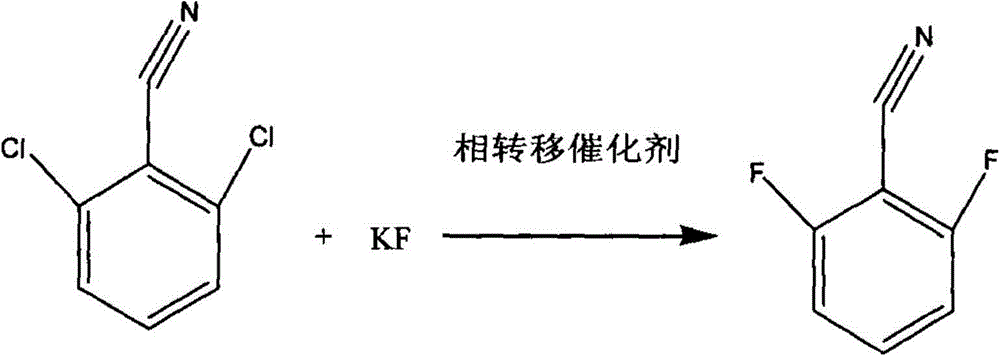
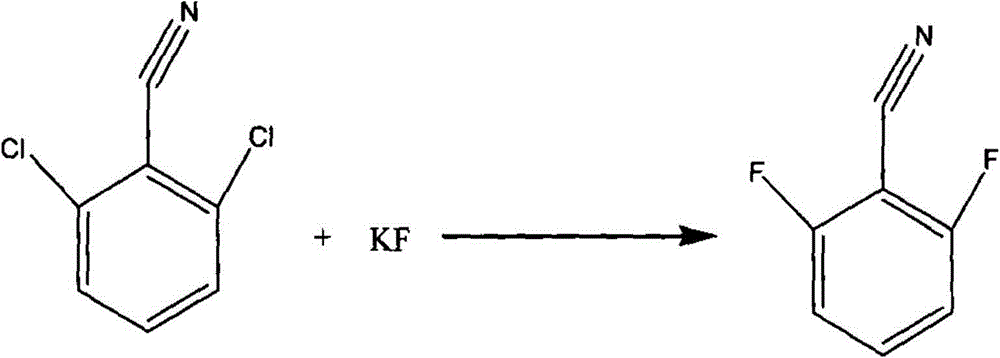
[0035] In a 1000ml mechanically stirred reaction flask equipped with a condenser and a water separator, 1,3-dimethyl-2-imidazolinone 450g, toluene 100g, 2,6-dichlorobenzonitrile 150g (content 99.5% , 0.868mol), N-ethylpyridine chloride 9g, stirred and heated to 110°C, refluxed for 2 hours, then continued to heat up to remove toluene, and recovered 99g of toluene.
[0036] Turn off the heating, lower the reaction temperature to 90°C, add 122g of anhydrous potassium fluoride (99% content, 2.08mol) into the reaction flask, heat to raise the reaction temperature to 150°C, keep the reaction for 15 hours, then turn off the heating.
[0037] Lower the reaction temperature to 40°C, filter with suction, transfer the filtrate to a rectifying bottle, turn on the vacuum, the vacuum degree is -0.1MPa, collect 105-108°C fractions, and obtain 118.8g of finished product 2,6-difluorobenzonitrile (content 99.5%, 0.85mol), yield 98%. The rectification raffinate is recovered and applied mechanic...
Embodiment 2
[0039] In a 1000ml mechanically stirred reaction flask equipped with a condenser and a water separator, 1,3-dimethyl-2-imidazolinone 450g, toluene 100g, 2,6-dichlorobenzonitrile 150g (content 99.5% , 0.868mol), 9g of N-propylpyridine chloride, stirred and heated up to 110°C, refluxed and separated for 2 hours, then continued to heat up to remove toluene, and recovered 99g of toluene.
[0040] Turn off the heating, lower the reaction temperature to 90°C, add 122g of anhydrous potassium fluoride (99% content, 2.08mol) into the reaction flask, heat to raise the reaction temperature to 150°C, keep the reaction for 15 hours, then turn off the heating.
[0041] Lower the reaction temperature to 40°C, filter with suction, transfer the filtrate to a rectifying bottle, turn on the vacuum, the vacuum degree is -0.1MPa, collect fractions at 105-108°C, and obtain 119g of finished product 2,6-difluorobenzonitrile (content 99.5 %, 0.852mol), yield 98.17%. The rectification raffinate is rec...
Embodiment 3
[0043] In a 1000ml mechanically stirred reaction flask equipped with a condenser and a water separator, dimethylsulfoxide, 100g of toluene, 150g of 2,6-dichlorobenzonitrile (content 99.5%, 0.868mol), N-chlorinated Ethylpyridine 9g, stirred and heated up to 110°C, refluxed to separate water for 2 hours, then continued to heat up to remove toluene, and recovered 99g of toluene.
[0044] Turn off the heating, lower the reaction temperature to 90°C, add 122g of anhydrous potassium fluoride (99% content, 2.08mol) into the reaction flask, heat to raise the reaction temperature to 150°C, keep the reaction for 15 hours, then turn off the heating.
[0045] Lower the reaction temperature to 40°C, filter with suction, transfer the filtrate to a rectifying bottle, turn on the vacuum, the vacuum degree is -0.1MPa, collect the fraction at 105-108°C, and obtain 119.2g of the finished product 2,6-difluorobenzonitrile (content 99.5%, 0.853mol), yield 98.34%. The rectification raffinate is rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com