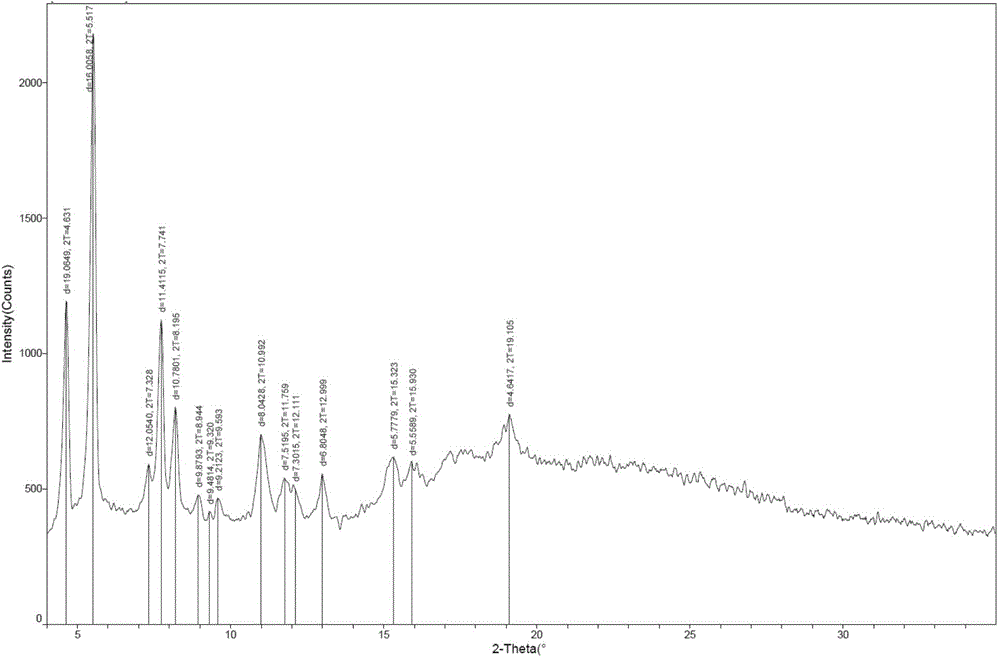
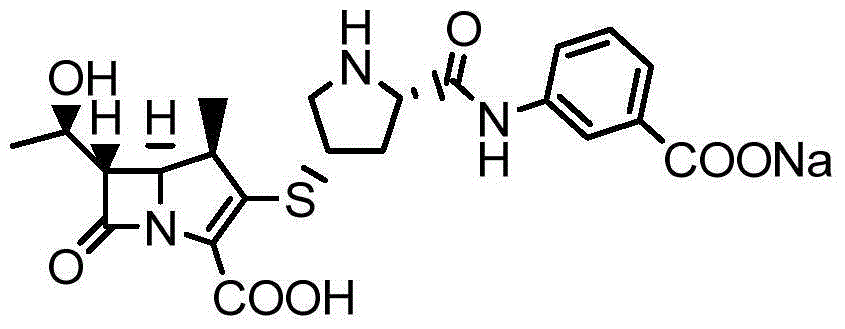
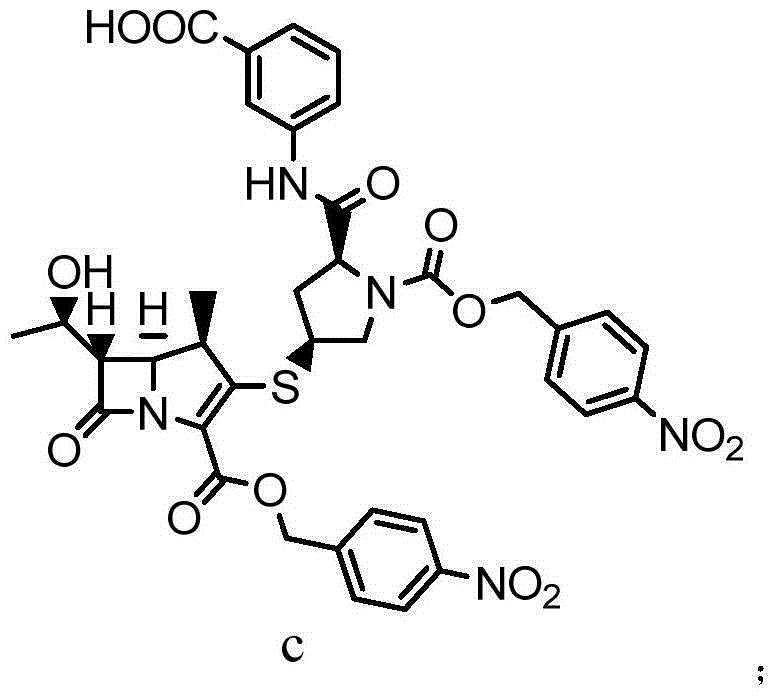
New monosodium ertapenem crystal form and preparation process thereof
A technology for the preparation of ertapenem, applied in the direction of organic chemistry, organic chemistry methods, etc., can solve the problems of affecting crystal form stability, small particle size of activated carbon, and degradation of ertapenem, so as to reduce moisture absorption and Degradation risk, low risk of product degradation, easy to filter effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of ertapenem monosodium salt crystal form
[0060]Put the double-protected base ertapenem (48g, 0.061mol) into a three-necked flask, add 300ml THF, stir to dissolve and clarify, then cool to about 0-5°C, add 600ml of 1% sodium bicarbonate solution dropwise, after dropping, put Pour the mixed solution into a 2000L autoclave, then add 12.0g of 10% palladium carbon, replace the reaction kettle with nitrogen, and then replace with hydrogen. After 5 hours, TLC detection was completed and the reaction was filtered, the filter cake was rinsed with 5ml of pure water, the filtrate was adjusted to PH=5-6 with acetic acid / methanol, then 500ml of isopropanol and 500ml of dichloromethane were added, and the layers were stirred for 10 minutes and separated. Add 300ml of n-butanol to the aqueous layer, stir and separate the layers, separate the aqueous layer and add methanol / n-propanol (200 / 100ml) mixture dropwise after filtration. After dropping, add ertapenem ...
Embodiment 2
[0061] Example 2: Preparation of ertapenem monosodium salt crystal form
[0062] Put the double-protected base ertapenem (48g, 0.061mol) into a three-neck flask, add 350ml THF, stir to dissolve and clarify, then cool to about 0-5°C, add 160ml of 5% sodium bicarbonate solution dropwise, after the drop is completed, put The mixture was poured into a 2000L autoclave, and then 10.0g of 10% palladium carbon was added. The reactor was replaced with nitrogen, and then replaced with hydrogen. After 5 hours, the TLC detection reaction was completed and filtered, the filter cake was rinsed with 5ml of pure water, the filtrate was adjusted to PH=5-6 with acetic acid / methanol, then 600ml of n-propanol and 700ml of dichloromethane were added, and the layers were stirred for 10 minutes and separated. Add 300ml of n-butanol to the aqueous layer, stir and separate the layers, separate the aqueous layer and add methanol / n-propanol (250 / 125ml) mixture dropwise after filtration. After dropping, ...
Embodiment 3
[0063] Example 3: Preparation of ertapenem monosodium salt crystal form
[0064] Put the double-protected base ertapenem (48g, 0.061mol) into a three-necked flask, add 350ml THF, stir to dissolve and clarify, then cool to about 0-5°C, add 250ml of 3% sodium bicarbonate solution dropwise, after dropping, put Pour the mixed liquid into a 2000L autoclave, then add 13.0g of 10% palladium carbon, replace the reaction kettle with nitrogen, and then replace with hydrogen. After 5 hours, after TLC detection, the reaction was completed and the filter cake was rinsed with 5ml of pure water. The filtrate was adjusted to PH=5-6 with acetic acid / methanol, then 600ml of n-propanol and 500ml of dichloromethane were added, and the layers were stirred for 10 minutes and separated. Add 300ml of n-butanol to the obtained water layer, stir and separate layers, separate the obtained water layer and drop into methanol / n-propanol (400 / 400ml) mixed solution after filtering. After dropping, add ertape...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diffraction angle | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com