A method for increasing the number of directional differentiation of umbilical cord blood megakaryotic progenitor cells
A technology for megakaryotic progenitor cells and directed differentiation, which is applied in the field of increasing the number of directed differentiation of umbilical cord blood hematopoietic stem cells to megakaryotic progenitor cells, can solve problems such as increased cost, pollution, and adverse effects on in vitro amplification efficiency, and achieves the goal of increasing the number of directed differentiation, The effect of high safety and less toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Umbilical cord blood was collected from healthy pregnant women and infants. Hepatitis B, hepatitis C, syphilis, AIDS, cytomegalovirus, TORCH, mycoplasma, chlamydia, G-6PD and thalassemia were all negative. The transport conditions of the specimens from collection to return to the blood bank were kept at 4-8°C. Perform directed differentiation of megakaryotic progenitor cells as follows:
[0034] 1. Preparation of resveratrol solution and universal medium
[0035] Resveratrol (≥99%, GC) was dissolved in a cell protection agent, and prepared into a resveratrol storage solution with a concentration of 10mmol / L.
[0036] The cell protection agent is a mixture obtained by mixing DMSO and Dextron at a volume ratio of 1:1.
[0037] Specific medium: Stemspan medium from STEMCELL company, IL-3 (20ng / mL), IL-6 (50ng / mL), SCF (50ng / mL) and TPO (50ng / mL) cytokines were added respectively.
[0038]Specific medium containing resveratrol: Add the above-prepared resveratrol storage...
Embodiment 2
[0059] Directed expansion of umbilical cord blood hematopoietic stem cells to megakaryotic progenitor cells with reference to the method of Example 1:
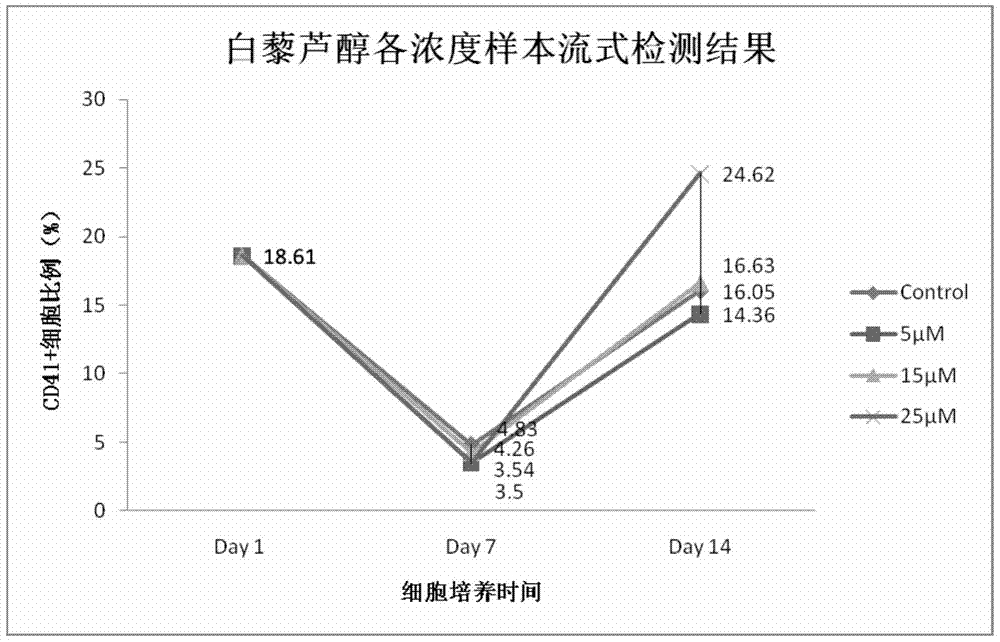
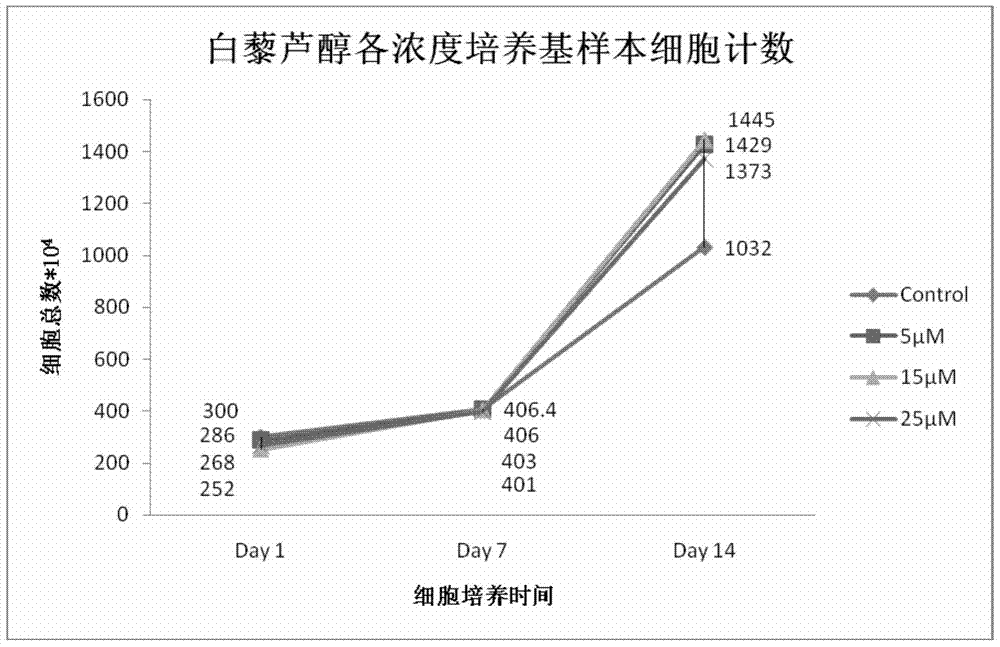
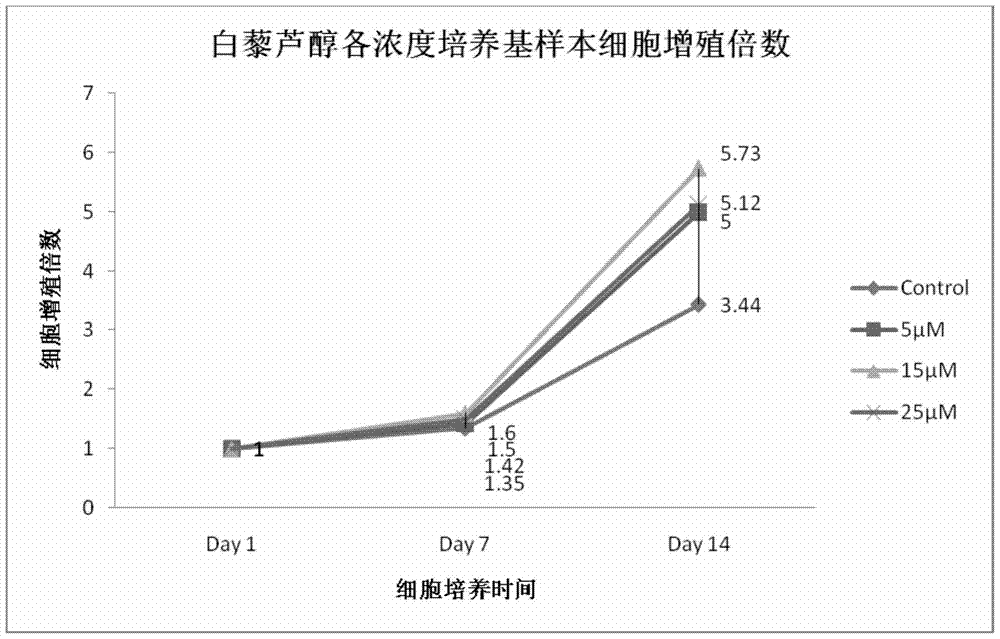
[0060] On the first day, the initial number of hematopoietic stem / progenitor cells in Control, 5 μmol / L, 15 μmol / L, and 25 μmol / L resveratrol samples were: 4.26×10 6 pcs / mL, 4.0×10 6 pcs / mL, 3.54×10 6 pcs / mL, 3.88×10 6 individual / mL.
[0061] On the 6th day, the number of hematopoietic stem / progenitor cells in Control, 5 μmol / L, 15 μmol / L, and 25 μmol / L resveratrol samples were: 3.34×10 6 pcs / mL, 3.73×10 6 pcs / mL, 4.27×10 6 pcs / mL, 4.46×10 6 cells / mL; detected by flow cytometry, in CD41 + In terms of cell ratios, the results were: 6.35%, 8.18%, 8.45%, and 8.54%, respectively.
[0062] On the 12th day, the number of hematopoietic stem / progenitor cells in Control, 5 μmol / L, 15 μmol / L, and 25 μmol / L resveratrol samples were: 15.57×10 6 pcs / mL, 16.36×10 6 pcs / mL, 17.64×10 6 pcs / mL, 11.39×10 6 cells / mL, compared with th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com