Patents
Literature
50 results about "Multinucleate giant cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multinucleated giant cells due to an infection. H&E stain. A giant cell (multinucleated giant cell, multinucleate giant cell) is a mass formed by the union of several distinct cells (usually macrophages), often forming a granuloma. It can arise in response to an infection, such as from tuberculosis, herpes, or HIV, or foreign body.
Immunity colloidal gold reagent for detecting IgG antibody of giant cells virus and its preparing method
The reagent includes the sample pad, the binding pad, the cellulose nitrate film, the water uptake pad and the PVC back cover. The sample pad, the binding pad are adhered to the one end with PVC backcovered in sequence. The cellulose nitrate film is adhered to the middle. The water uptake pad is adhered to another end of the reagent. The binding pad is coated with the label of albumen A (or rabbit anti human IgG)-colloidal gold. The cellulose nitrate film is coated with the specificity surface film antigen of giant cells virus and chickens anti albumen A (or sheep anti rabbit IgG). The reagent features high specificity and sensitivity, simple and fast. The reagent is applicable to the detection in site within 30 minutes for the full testing procedure.
Owner:博顿生物检验技术(杭州)有限公司
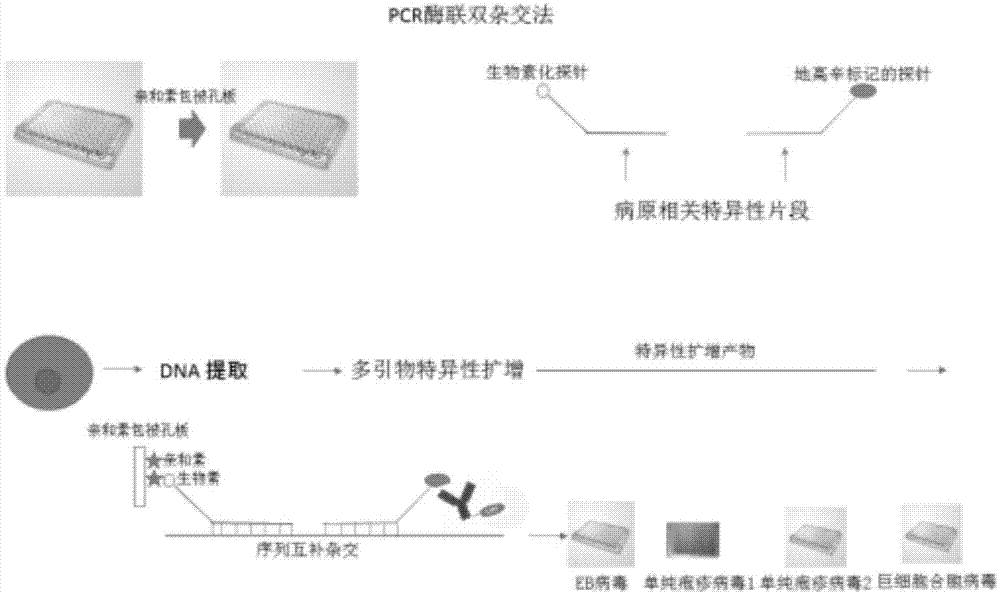
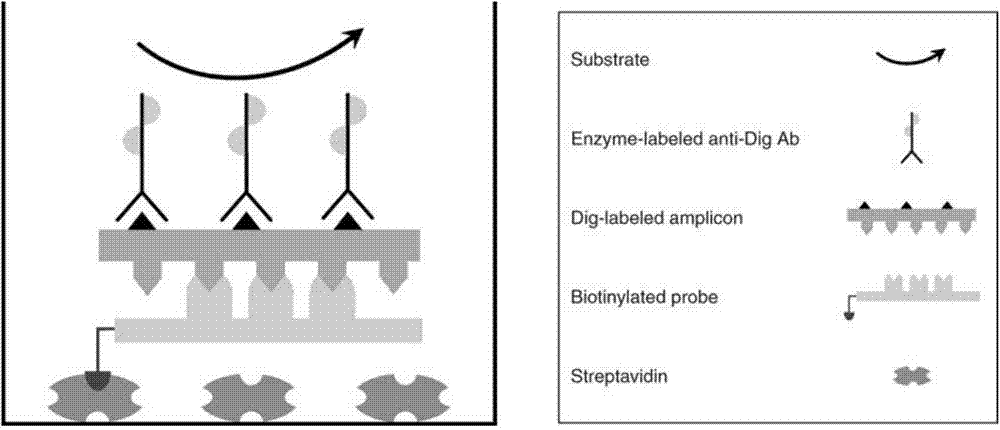
Method for detecting pathogenic microorganisms by using PCR (polymerase chain reaction) enzyme-linked double-cross method
ActiveCN104726606AFlexible and convenient to useFlexible solid phase junctionMicrobiological testing/measurementDNA hybridisationMultinucleate giant cell
The invention provides a method for detecting pathogenic microorganisms by using a PCR (polymerase chain reaction) enzyme-linked double-cross method, belongs to the technical field of clinical medical microorganism detection, and particularly relates to a synchronous qualitative and quantitative detection method for various microorganisms. In the method, DNA (deoxyribonucleic acid) amplification, DNA hybridization and enzyme-linked immunosorbent assay reaction are used, a solid-phase surface enveloping technology is adopted, a specificity conservative segment is amplified, an amplified product is combined to a capturing probe and is adsorbed to a solid-phase surface, far-end hybridization of an enzyme-linked probe is carried out, and a quantitative color product is generated by specific antibody recognition and reaction of enzyme and a substrate. The detected pathogenic microorganisms comprise EB (Epstein Barr) viruses, giant cell and cell viruses, herpes simplex viruses 1 and herpes simplex viruses 2. Various microorganisms of a clinical sample are detected simultaneously, and four types of viruses are screened by a reaction. The method has the advantages that the time and the cost are saved, application is flexible, the repeatability, the stability, the sensitivity and the specificity are high, and the method is simple and is easy to implement. Moreover, the method is suitable for widely detecting pathogenic microorganisms and is particularly suitable for a basic medical institution.
Owner:NANJING AIPEIJIE BIOLOGICAL TECH CO LTD
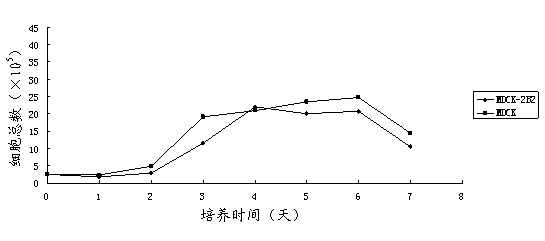
Non-tumorigenic MDCK cell line used for amplifying influenza viruses and screening method thereof
ActiveCN104059873AReduced tumorigenicityIncreased tumorigenicityMicroorganism based processesArtificial cell constructsScreening methodMorphologic change
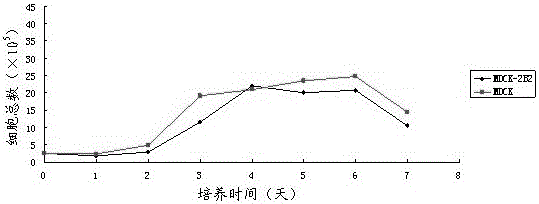
The invention discloses a non-tumorigenic MDCK cell line used for amplifying influenza viruses and a screening method thereof. The cell line is MDCK-2B2 with an accession number of CCTCC 201323. The invention provides the non-tumorigenic MDCK-2B2 cell line and a subclone cell line thereof; and the cell line can grow into adherent cells, and / or has an epithelioid form and / or can support duplication of a variety of viruses, and is capable of amplifying viruses with high titers. The invention also provides the screening method for the non-tumorigenic cell line. The growth speed and morphologic changes of cells can be used to assist in determination of tumorigenicity; i.e., simple-pore cells with a slow growth speed have substantially reduced tumorigenicity, and occurrence of giant cells enables tumorigenicity to be substantially increased; during large-scale screening of non-tumorigenic cell lines, such an auxiliary determination method can shorten time and save cost.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
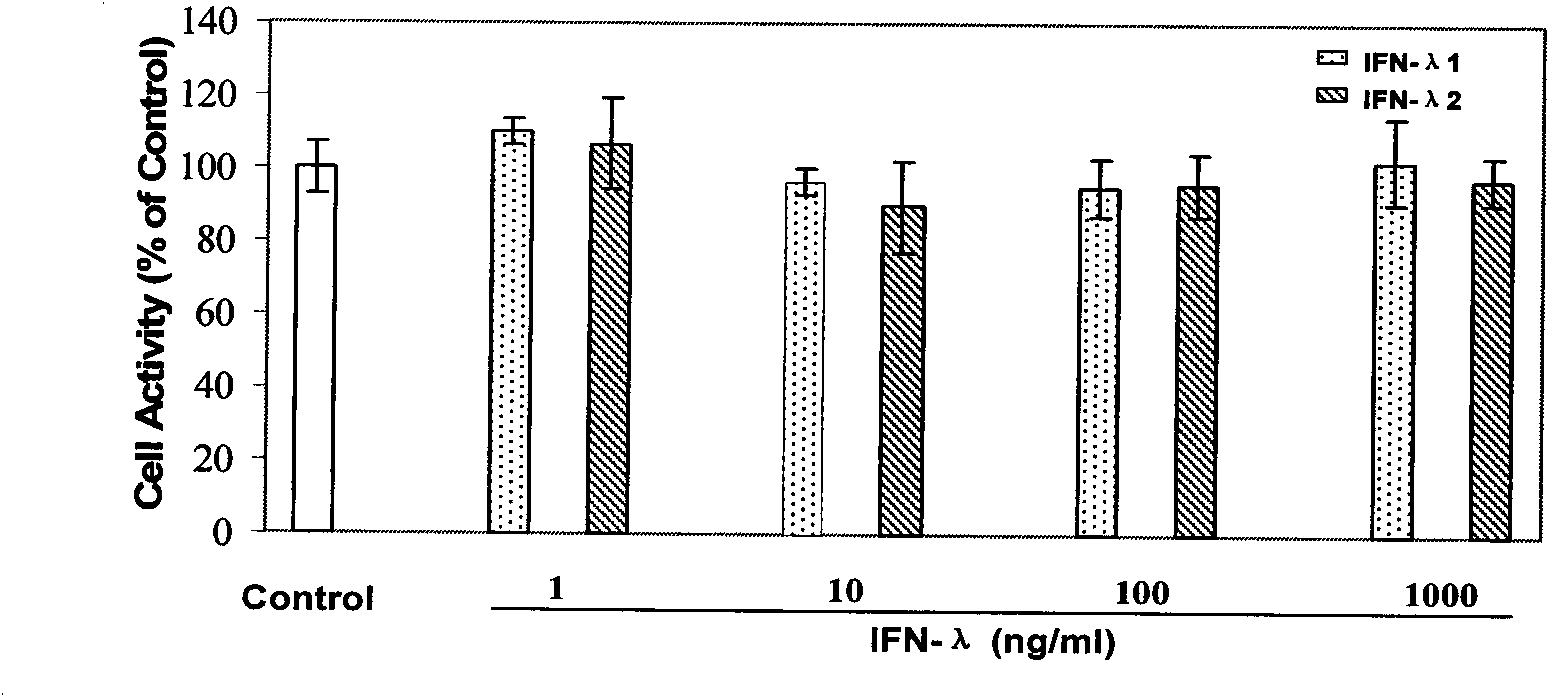
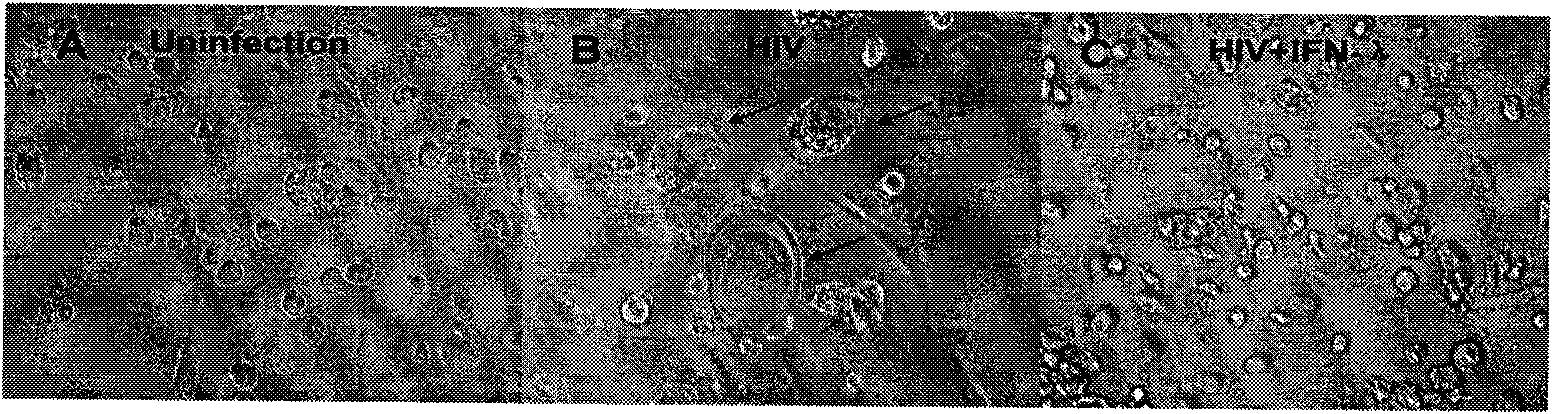
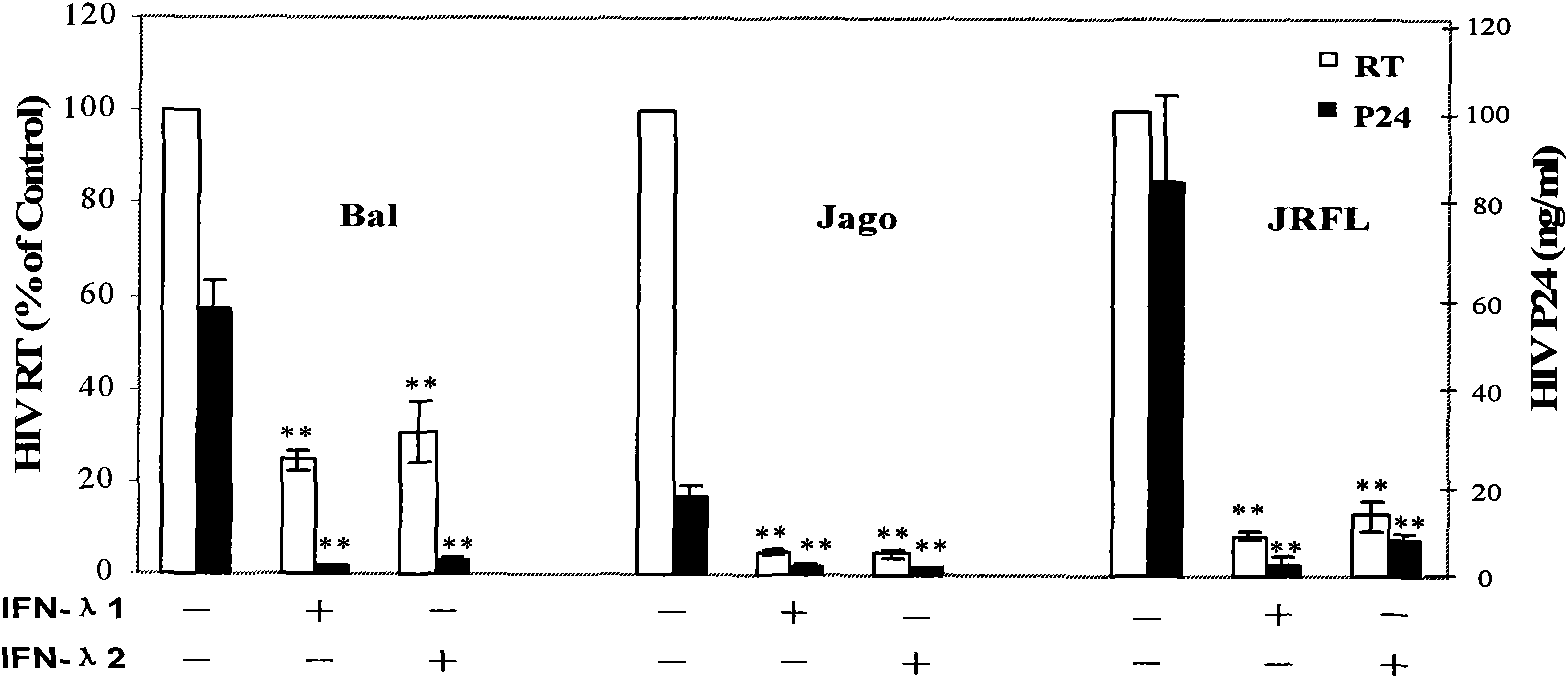
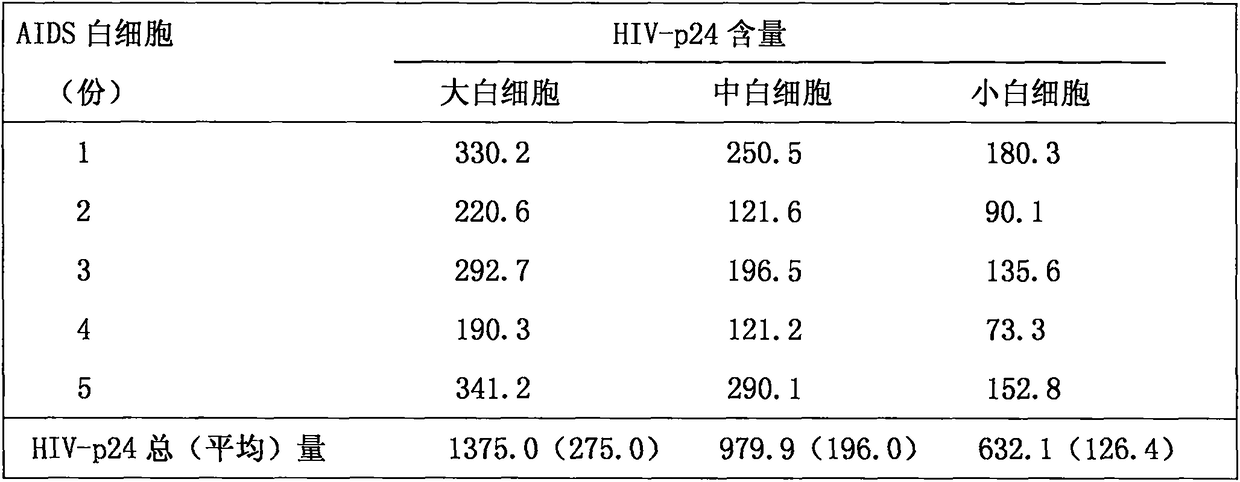
Application of Lambda interferon in resisting human immunodeficiency virus
InactiveCN101574515AInhibition of replicationAvoid infectionPeptide/protein ingredientsAntiviralsSerum igeAbnormal macrophage
The invention discloses an application of Lambda interferon in resisting human immunodeficiency virus. IFN-Lambda resisting HIV infection can inhibit the infection and replication of HIV-1 in human macrophage, which is presented by the following processes: (1) IFN-Lambda1 / Lambda2 differentiates mature human macrophage through pretreatment, then HIV-1R5 type strain respectively infects the cells, a DMEM culture medium is used for washing and then a DMEM culture medium containing 10 percent of fetal calf serum is added for culture; and (2) after infection, the formed multinucleate giant cells of IFN-Lambda treatment group are rather less than those of a virus contrast group, and the HIV-1 reverse transcriptase and envelope protein P24 in culture supernatant fluid of the IFN-Lambda treatment group are detected to be rather less than that of the virus contrast group. After infection, the HIV-1RT in the culture supernatant fluid is respectively detected. The effect of inhibiting the HIV-1 is obvious after infection. The IFN-Lambda treatment group has obvious time and dose-effect relationship in inhibiting the activity of HIV-1RT after infection. The function of IFN-Lambda in resisting the HIV-1 is similar to that of other interferons and has the antivirus effect. The IFN-Lambda shows obvious effect of inhibiting the replication of virus as regards to the human macrophage before, in and after the infection of the HIV-1 virus.
Owner:WUHAN UNIV
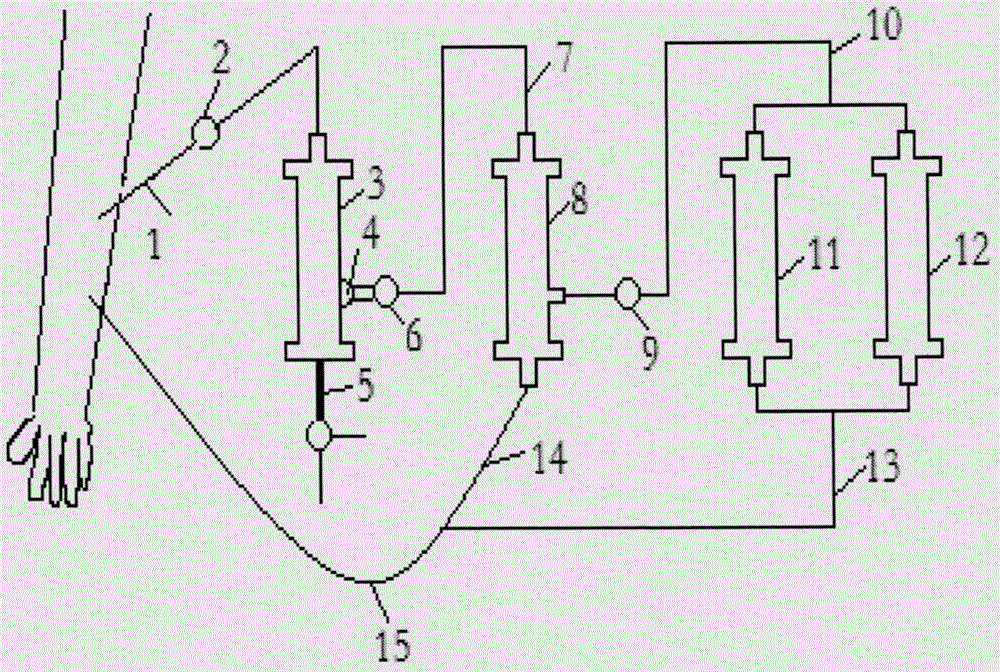
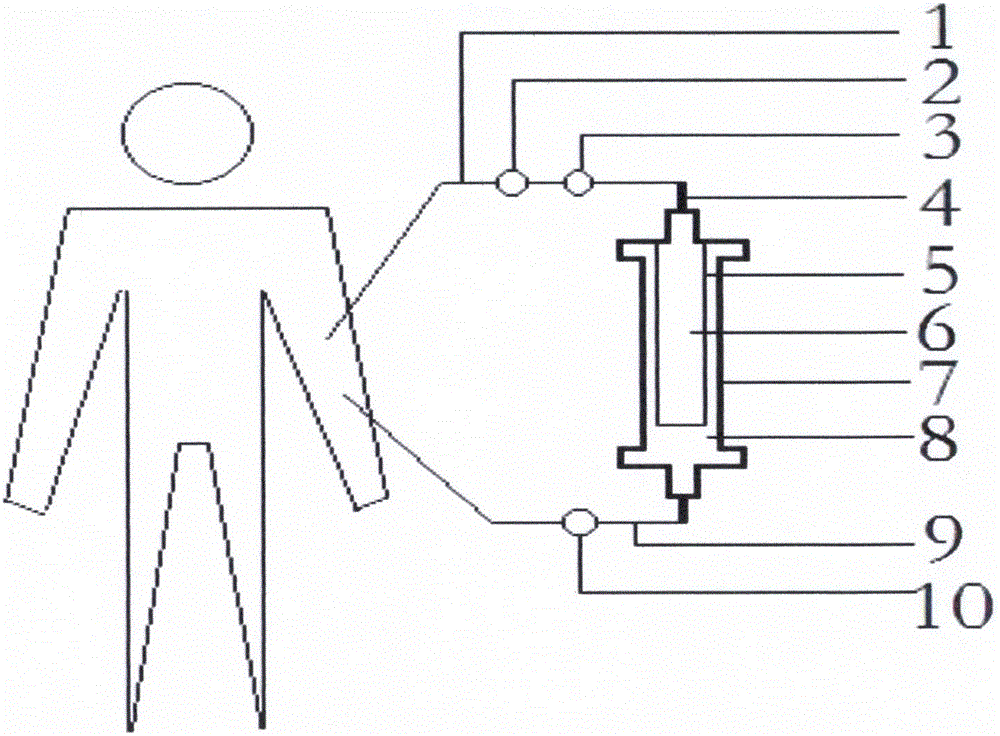
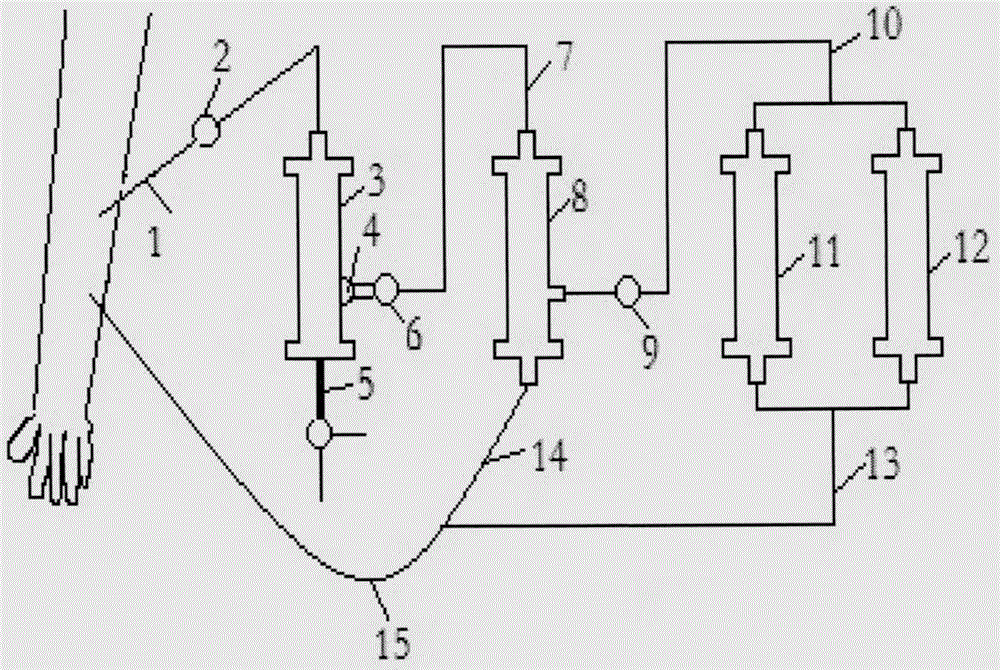
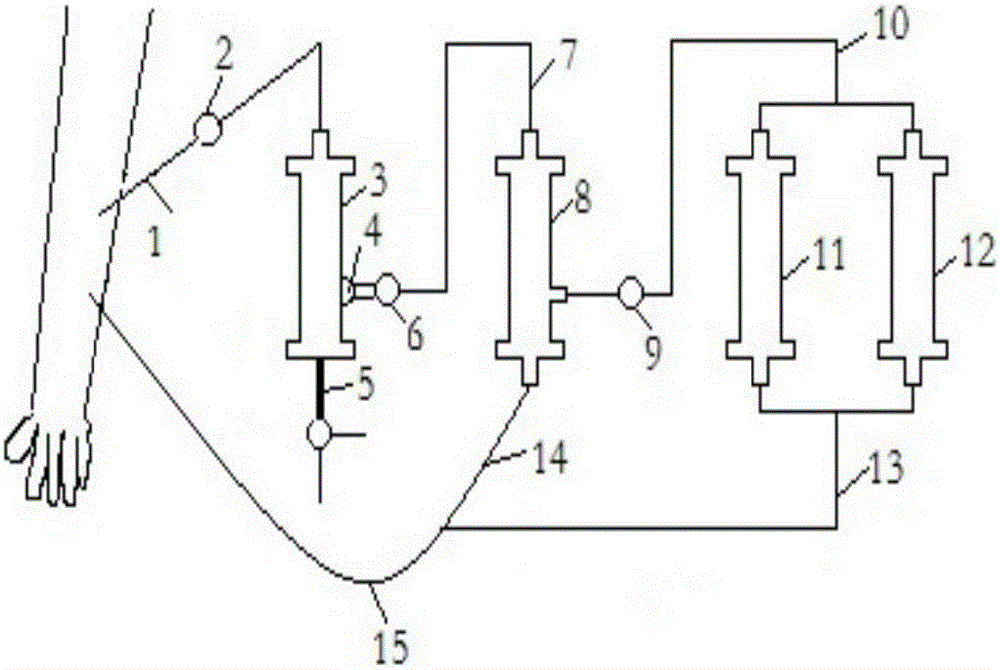
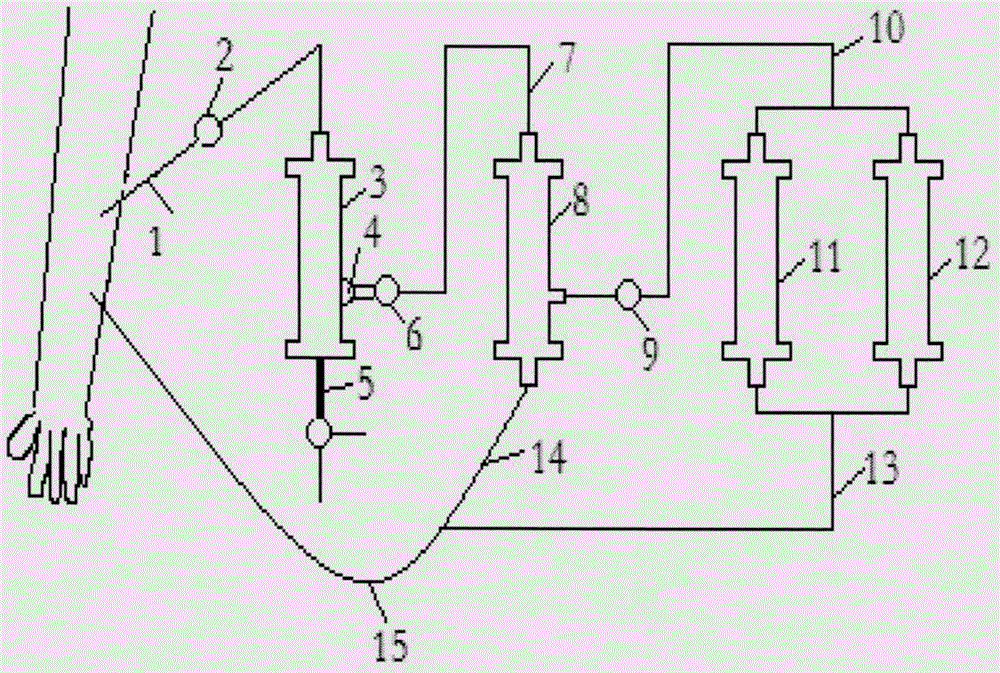
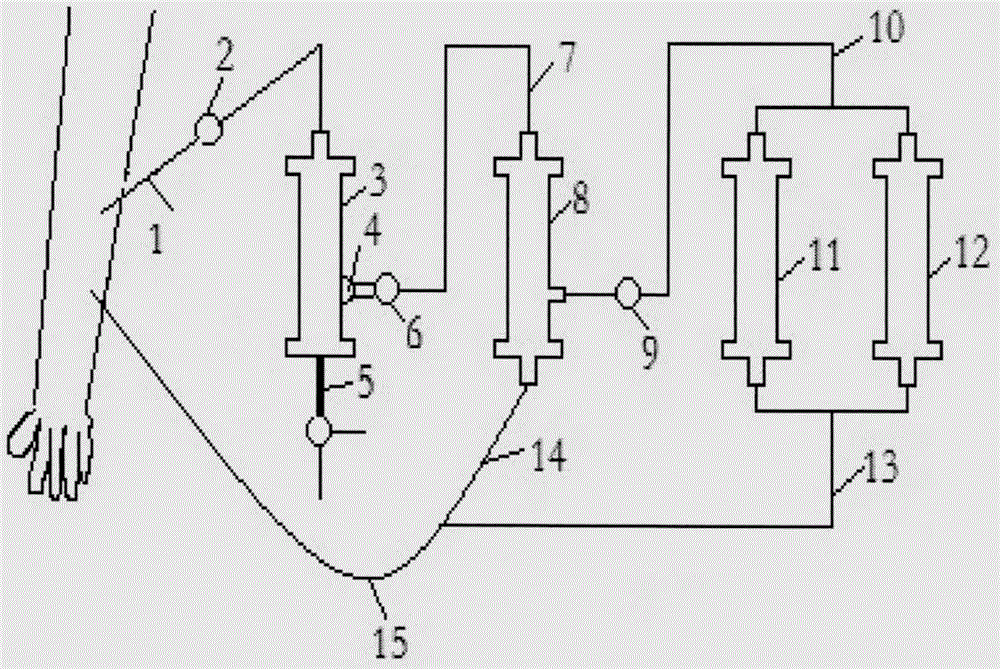
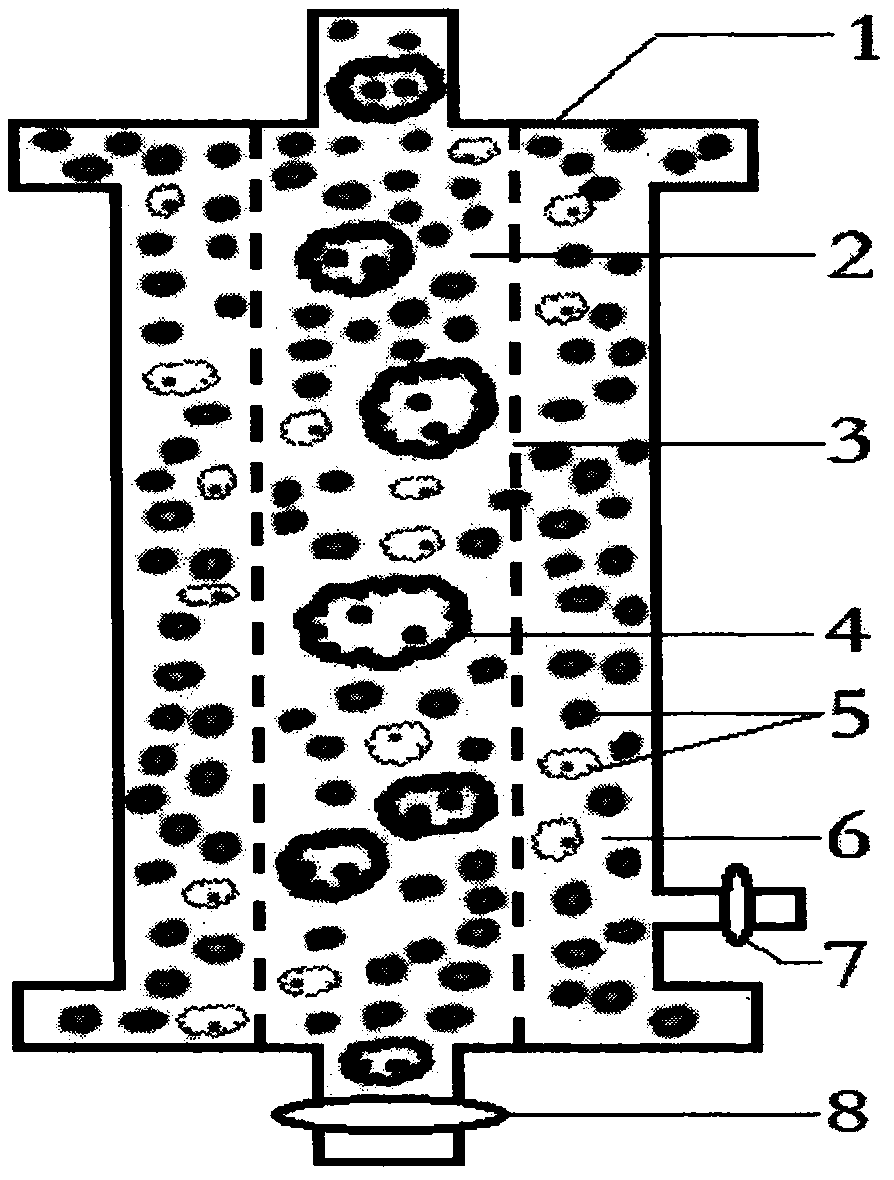
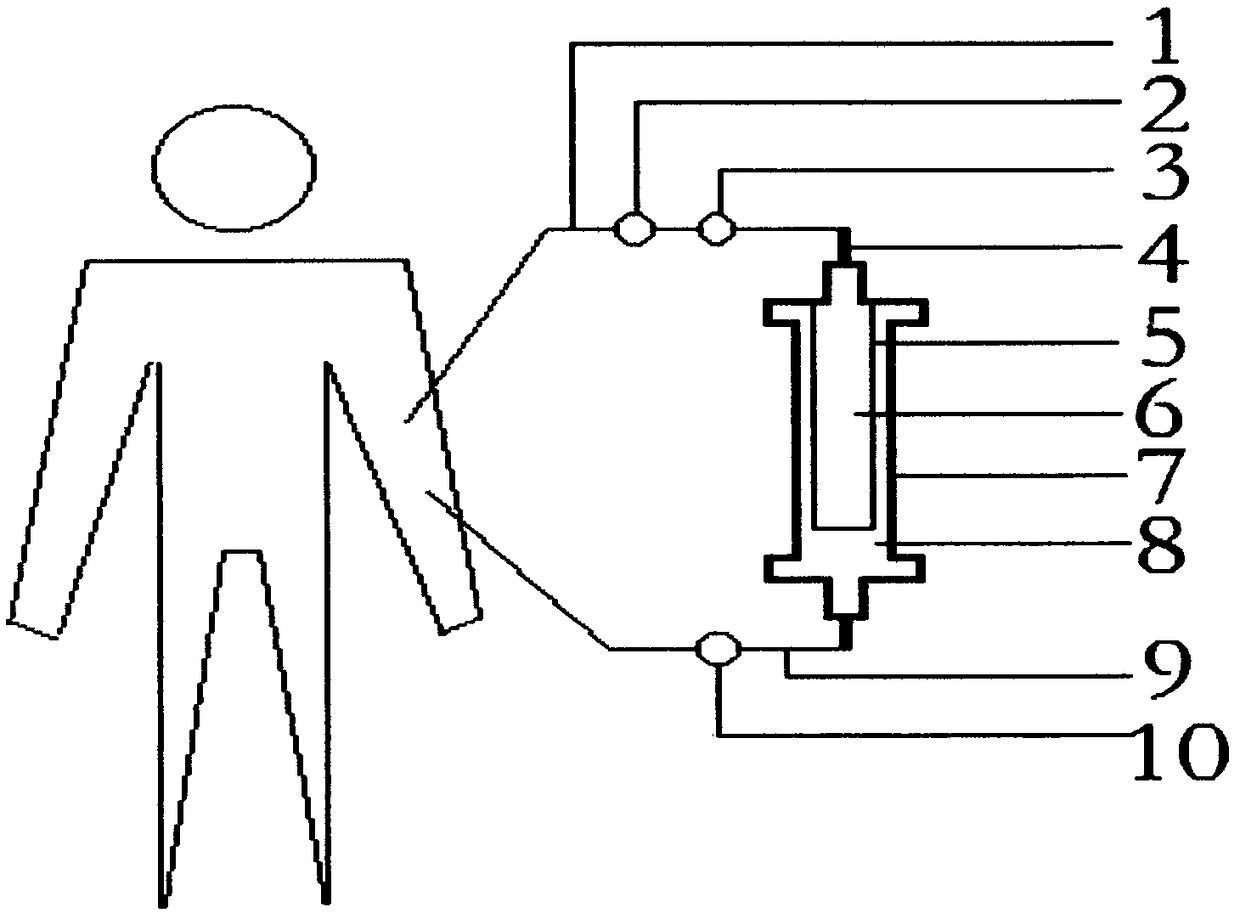
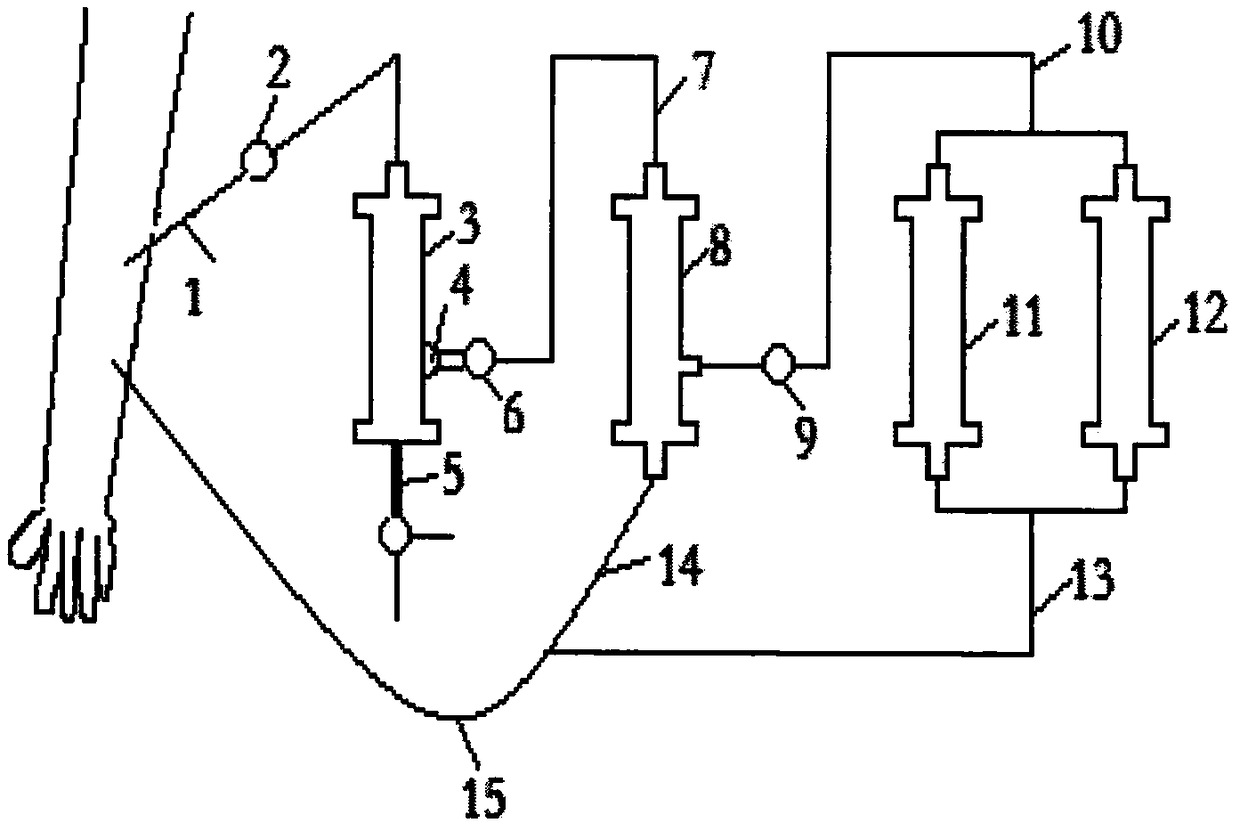
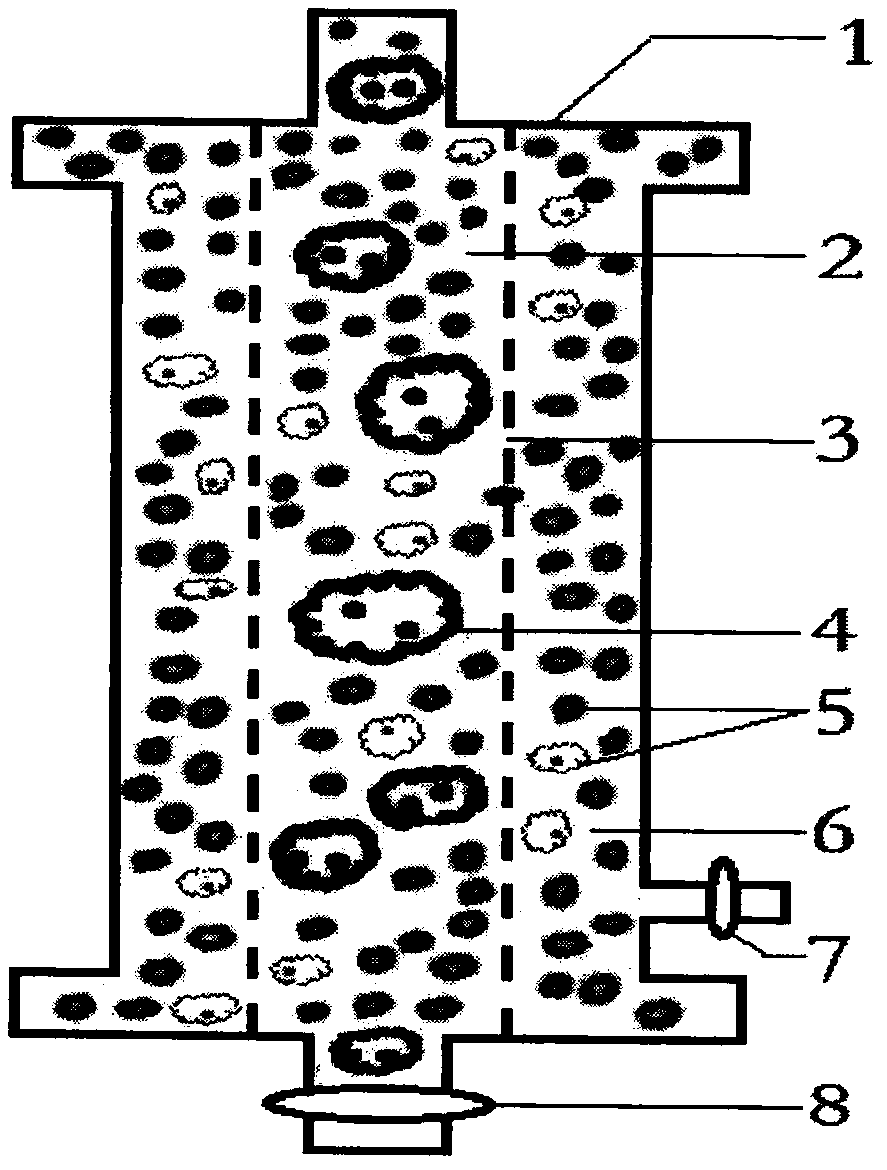
AIDS immunoadsorption therapy apparatus
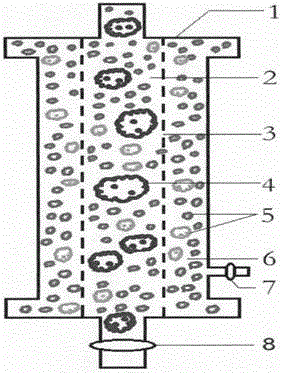
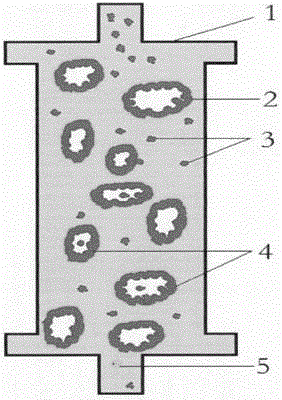
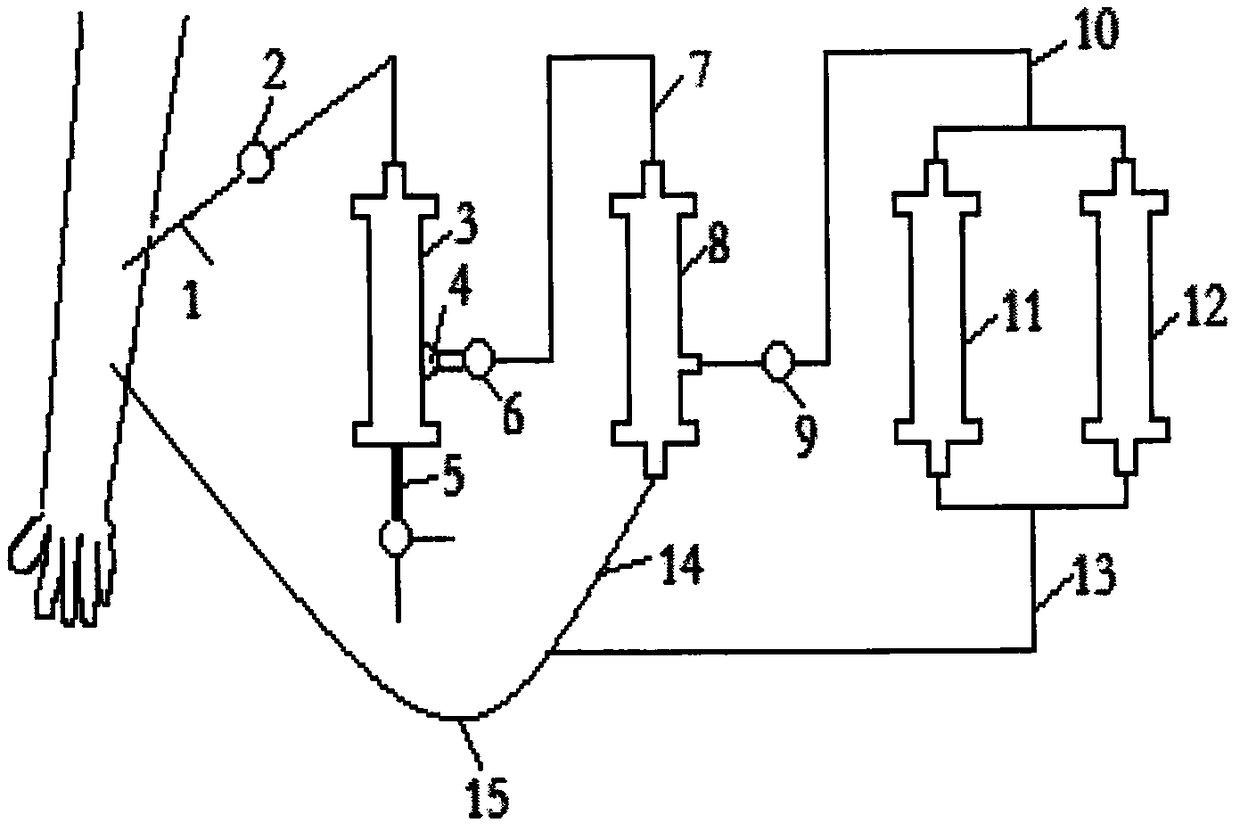
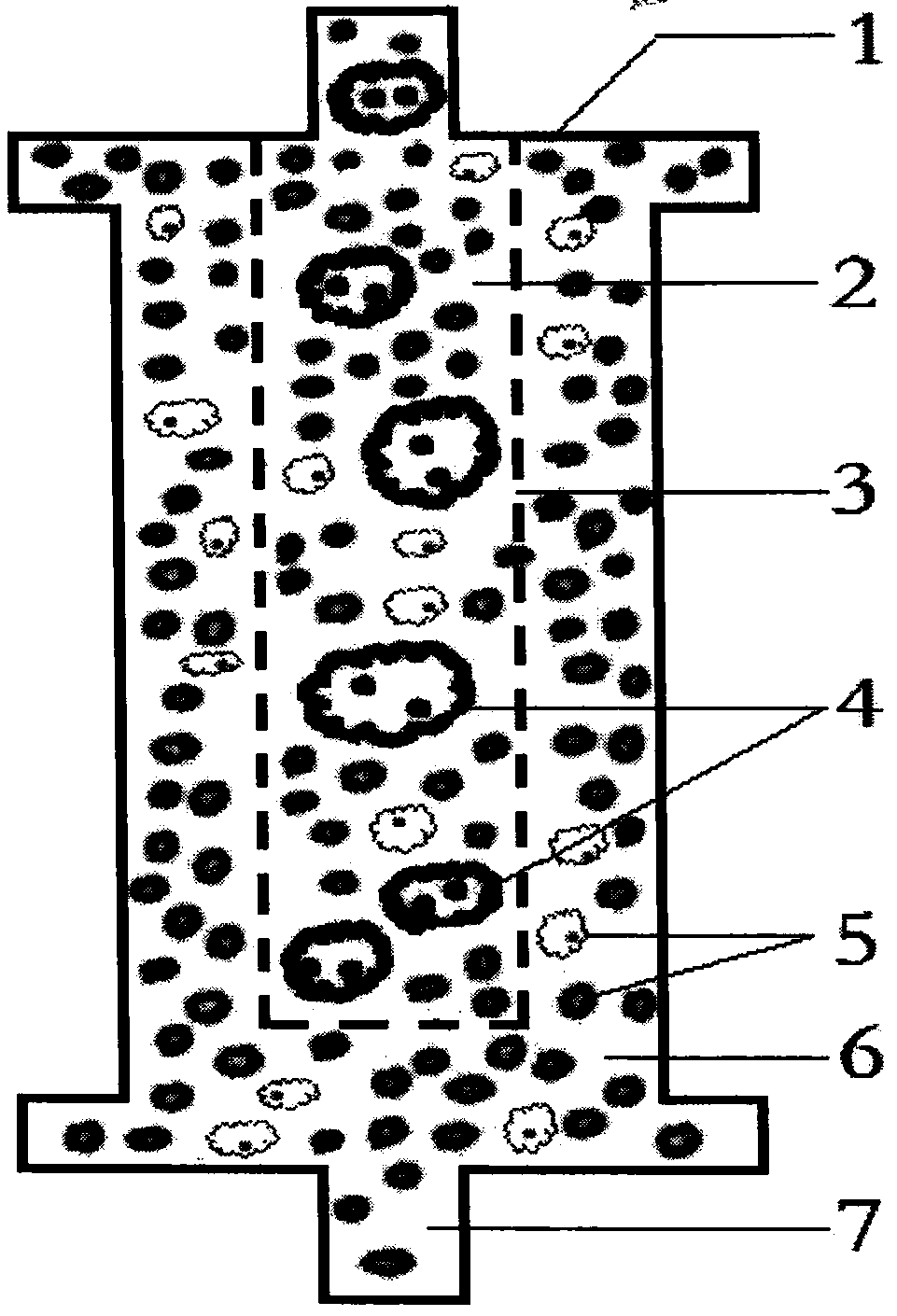
The invention discloses an AIDS immunoadsorption therapy apparatus, which is applicable to the medicine field. The AIDS immunoadsorption therapy apparatus is characterized in that blood and plasma separators, by which plasma, individual blood cells and multinuclear giant cells which are formed due to HIV boarding, are prepared; by virtue of a hybridoma technique and an exogenous gene transfection technique, a hybridoma macrophage cell line and a CD4+T cell line, which can bond HIV, are prepared, the cell lines, as adsorbing agents, are prepared in cell cryopreservation liquid, and the cell lines are filled with high polymer materials, so that adsorbers are prepared; the adsorbers, together with the separators, function as key parts of an extracorporeal circulation device; when blood passes through the blood separator, the multinuclear giant cells which contain the HIV are filtered out, subsequently, when the plasma, which is separated by virtue of the plasma separator, passes through the purifier, HIV in the plasma is adsorbed and scavenged by virtue of a purifying agent, the purified plasma joins with the individual blood cells which are separated by virtue of the plasma separator, and then the plasma is returned back, so that the purpose of scavenging the HIVs inside and outside the blood cells is achieved.
Owner:翁炳焕
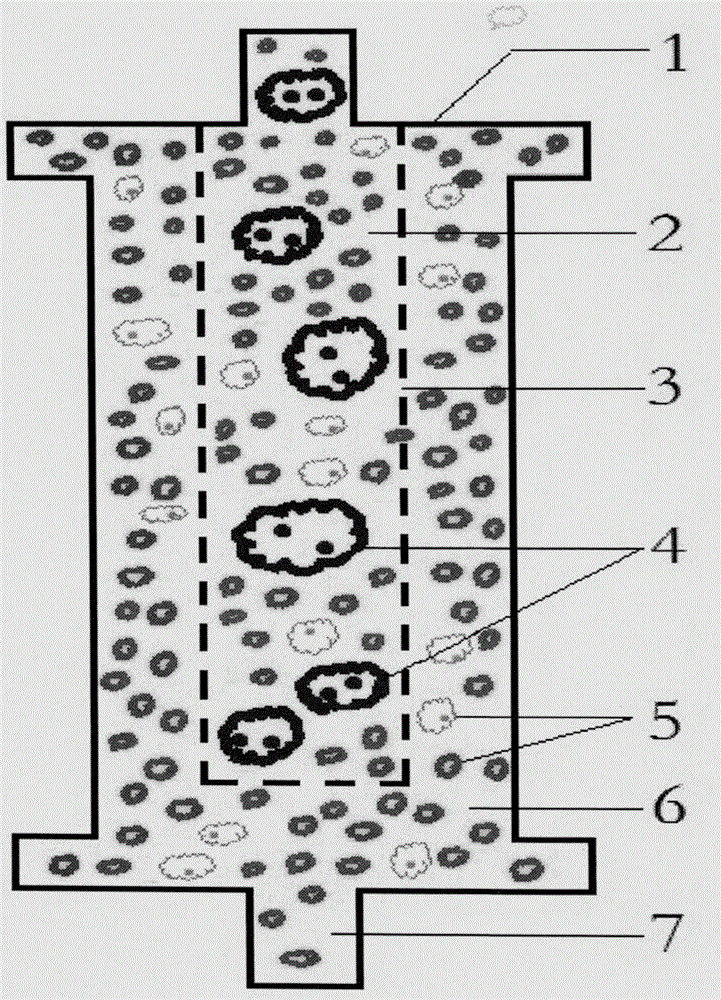
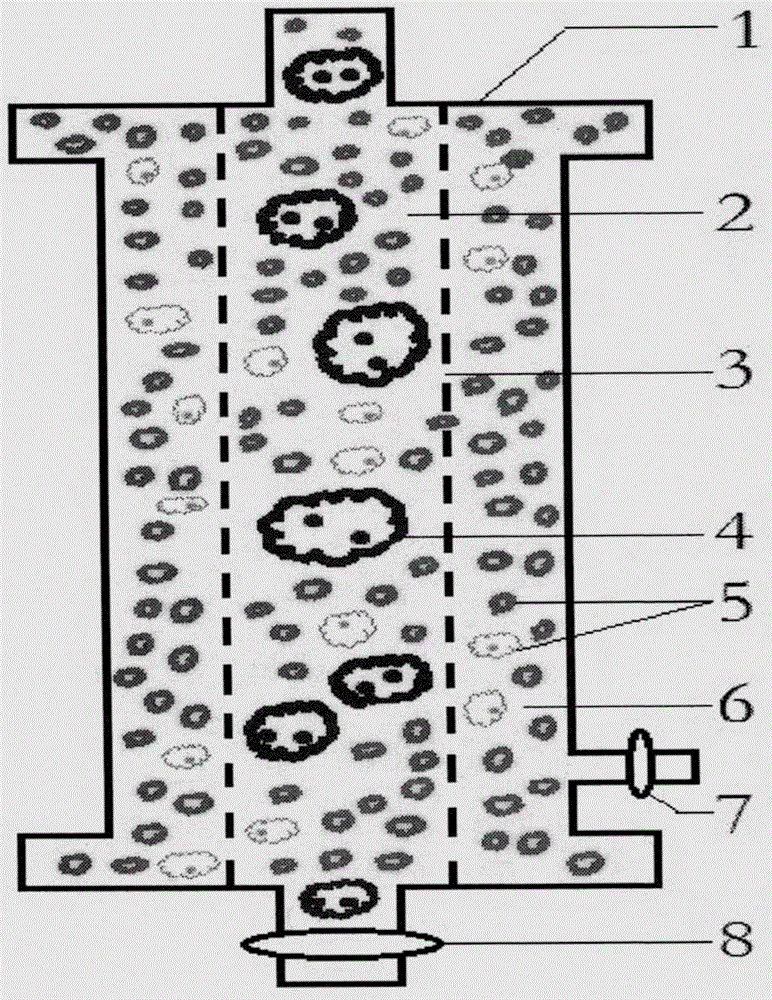
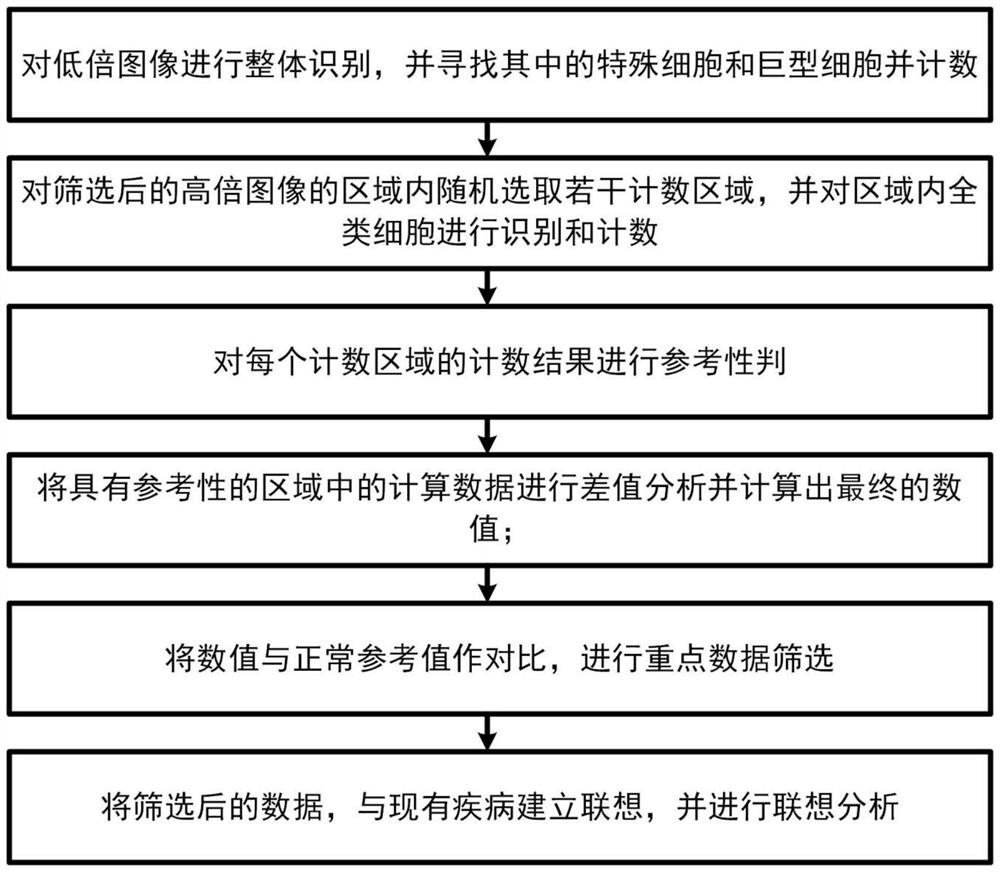
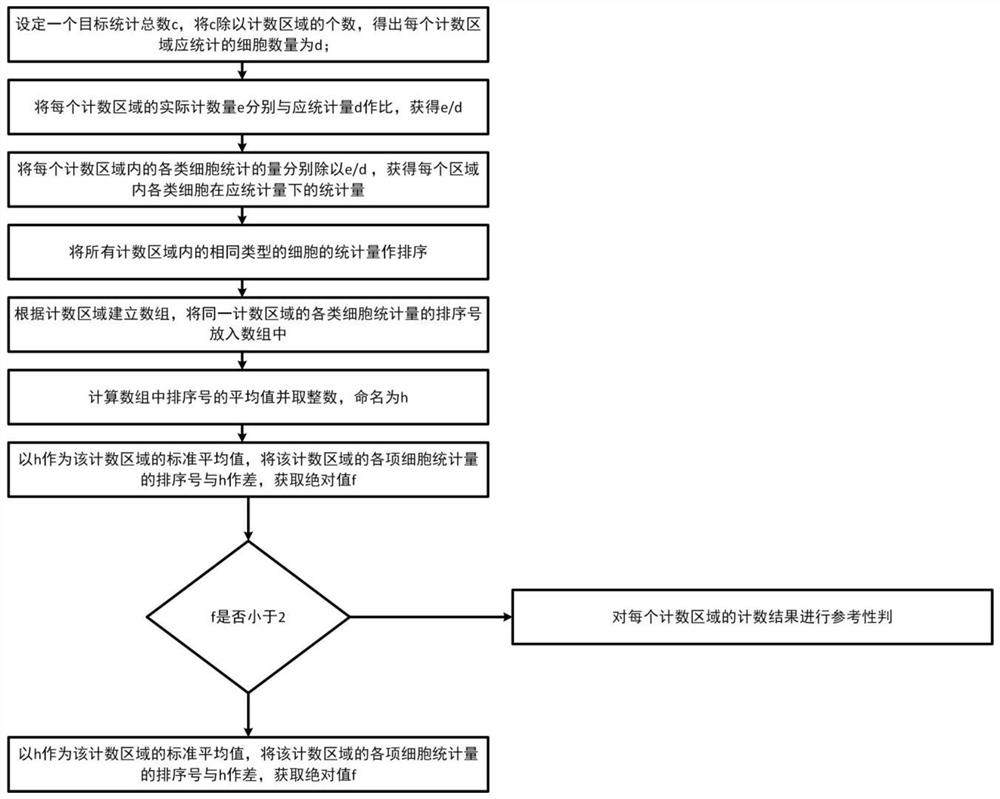
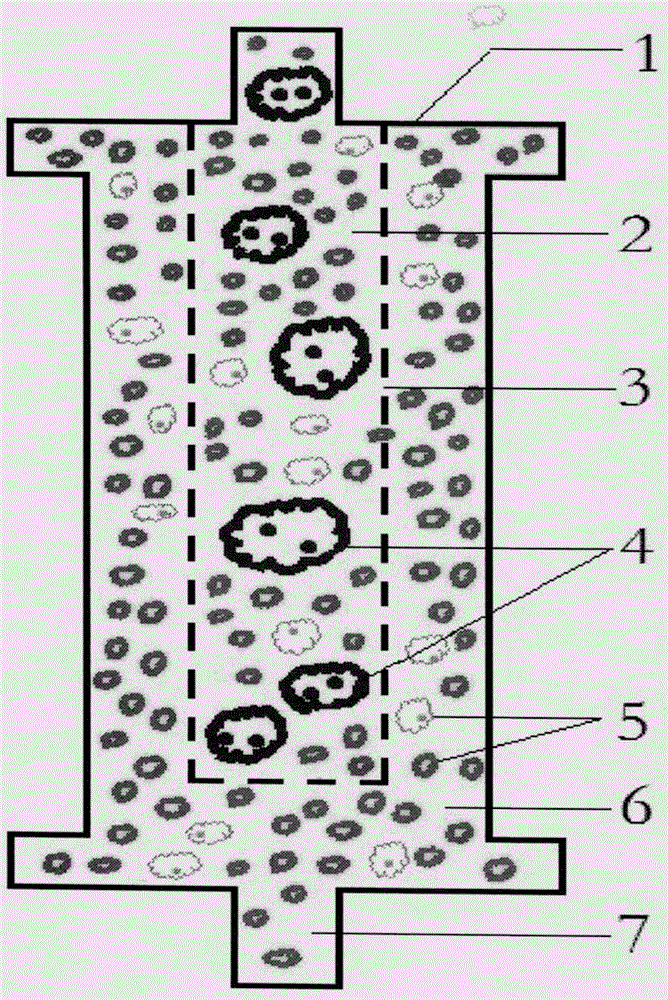
Counting and analyzing method of automatic bone marrow cell morphology detection system
The invention belongs to the field of bone marrow cell morphology detection, and particularly relates to a counting and analyzing method of an automatic bone marrow cell morphology detection system. According to the technical scheme, the counting and analyzing method of the automatic bone marrow cell morphology detection system comprises the following steps: S1, recognizing low-magnification images integrally, and searching and counting special cells and giant cells in the low-magnification images; and S2, randomly selecting a plurality of counting areas in the areas of the screened high-powerimages, and identifying and counting all kinds of cells in the areas; and S3, performing reference judgment on the counting result of each counting area. According to the invention, multi-point sampling is adopted, and then representative analysis is performed on the data of each sampling point, so that the data of each sampling point has reference. Then, all the data with the reference samplingpoints are subjected to weighted calculation, so that the statistical data is closer to a real value, and the final data is more accurate in pointing.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Common expression CTLA4Ig and CTLA4 recombinant adenovirus carrier, recombinant adenovirus and uses thereof
The invention discloses an IRES-mediated recombinant adenovirus vector and the recombinant adenovirus for co-expressing CTLA4Ig and CTLA4. The preparing method thereof comprises the steps: a CTLA4Ig gene, an IRES gene and a CTLA4 gene are inserted sequentially into the downstream of a giant cell virus promoter in a pAdTrack-CMV shuttle vector, a recombinant shuttle vector carrying the CTLA4Ig gene, the IRES gene and the CTLA4 gene is then obtained, and the recombinant shuttle vector is recombined in homologous with a virus backbone vector pAdeasy-1 to obtain the recombinant adenovirus vector for co-expressing the CTLA4Ig and the CTLA4; and the recombinant adenovirus expression vector transfects packaging cells (293 cells of human embryo kidney) to obtain recombinant adenovirus, and mammalian cells are infected by the recombinant adenovirus in vitro, thereby ensuring purpose cells with immunological suppression function. The recombinant adenovirus can play an important role in the medical field, especially in the organ transplanting field, thereby having extensive application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
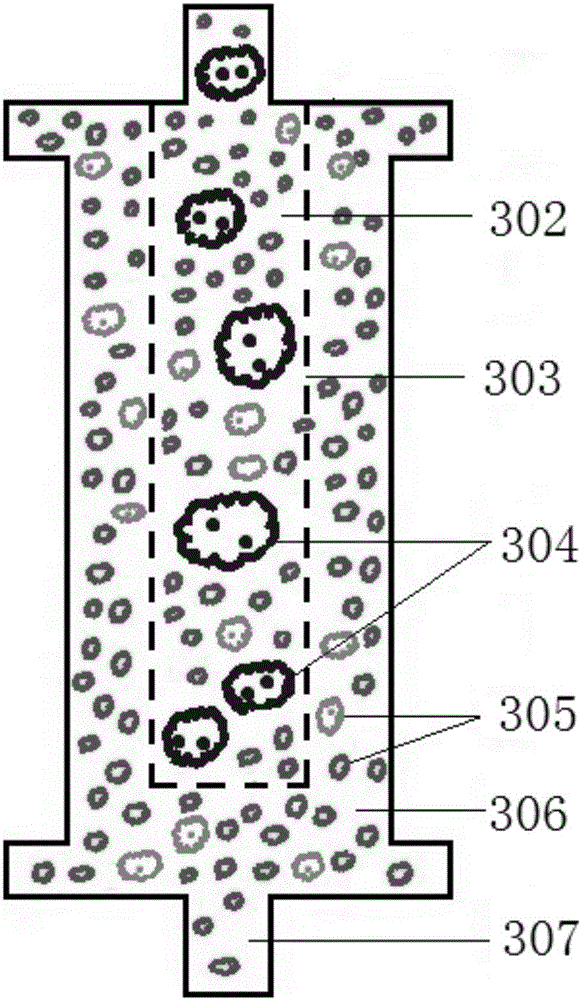
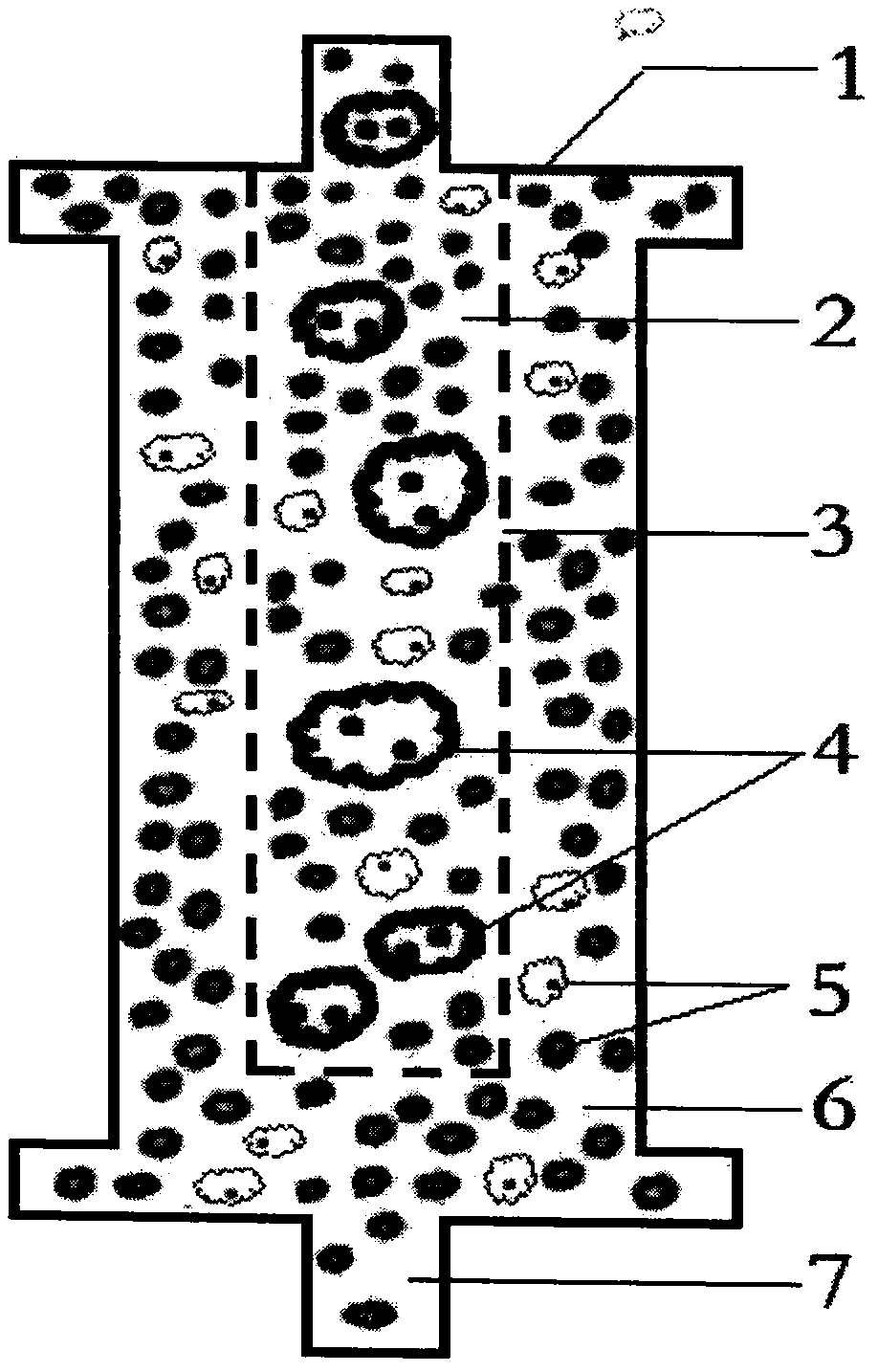
Acquired immune deficiency syndrome (AIDS) infected cell separator
ActiveCN106267410ATo achieve the therapeutic purpose of immune reconstructionOther blood circulation devicesExtracorporeal circulationInfected cell
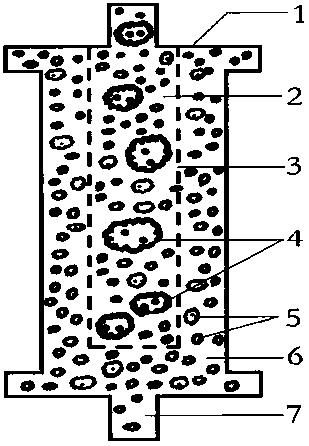
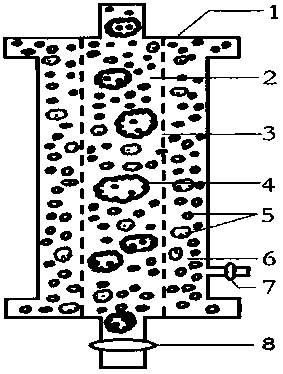
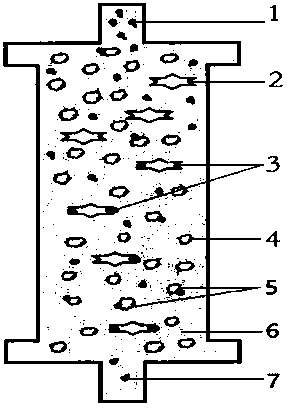
The invention relates to an acquired immune deficiency syndrome (AIDS) infected cell separator and belongs to the field of biomedicine. The AIDS infected cell separator is characterized in that a cell separator, through which a single blood cell and chemical components can pass, but a large-volume cell formed by combining two or more blood cells cannot pass, has pore diameter being 150 to 250 microns, 50 to 150 microns, 15 to 40 microns, 8 to 15 microns, 5 to 8 microns, 3 to 5 microns and 1 to 2 microns and is correspondingly calibrated as I-VII type, is prepared from a high-biocompatibility material; an HIV (human immunodeficiency virus) gp120 antibody and a CD4 antibody are prepared; gp120 on the surface of the HIV infected cell can be adhered to another CD4+ cell by itself or under the effects of the gp120 antibody or the CD4 antibody to form a large-volume multinuclear giant cell; the I-II type separators are selected to separate ultra-large volume fungi, spirillum and tumor cells; the III-VII type separators are selected to separate HIV infected large-volume multinuclear giant cells, multicellular polymers and CD4+ cells susceptible to HIV infection; the AIDS infected cells are separated and eliminated in extracorporeal circulation formed by the separator and other parts.
Owner:翁炳焕 +1
AIDS biological cellular immunotherapy apparatus
The invention discloses an AIDS biological cellular immunotherapy apparatus, and belongs to the field of medicine. The AIDS biological cellular immunotherapy apparatus is characterized in that blood and plasma separators, by which plasma, individual blood cells and multinuclear giant cells formed due to HIV parasitism are prepared; SV40LTag-pcDNA3.1 (-) and pLXSNneo-hTERT recons are constructed; combined transfection is conducted, so as to cause macrophage immortalization; the macrophage, after being subjected to amplification, is prepared in a cell cryopreservation solution until a cell concentration is 80%, and then, a purifier, which is made from a high polymer material, is poured with cells, wherein macrophage cell lines take an effect on phagocytizing HIV; the purifier and the blood and plasma separators constitute a key extracorporeal circulation device; when blood passes through the blood separator, the multinuclear giant cells which contain the HIV are filtered and removed, and subsequently, when plasma, which is separated by virtue of the plasma separator, passes through the purifier, the HIV in the plasma is phagocytized by the macrophage; and the purified plasma is mixed with the individual blood cells separated by virtue of the plasma separator, and then, the mixed plasma and individual blood cells are conveyed back, so that the purification treatment purpose of clearing the HIV inside and outside the blood cells is achieved.
Owner:ATTACHED OBSTETRICS & GYNECOLOGY OSPITAL MEDICALCOLLEGE ZHEJIANG UNIV +1
Four causative agent differential immunoglobulin G antibody and affinity index testing kit
A reagent kit of specificity IgG and its affine index detection is prepared by planting giant cell virus AD169 plant, herpes simplex virus I type SM-44 plant, herpes simplex II type G pland on diploid cell of human embryo lungs, planting nettle rash virus Gos plant, bowworm RH plant on Vero cell, obtaining antigen Via developing, encapsulating polystrene ELISA reaction plate after tiration and purification to be certain concentration as well as preparing specimen dilution liquid developer solution, concentration cleaning liquid and so on.
Owner:国家人口和计划生育委员会出生缺陷干预工程技术中心 +1
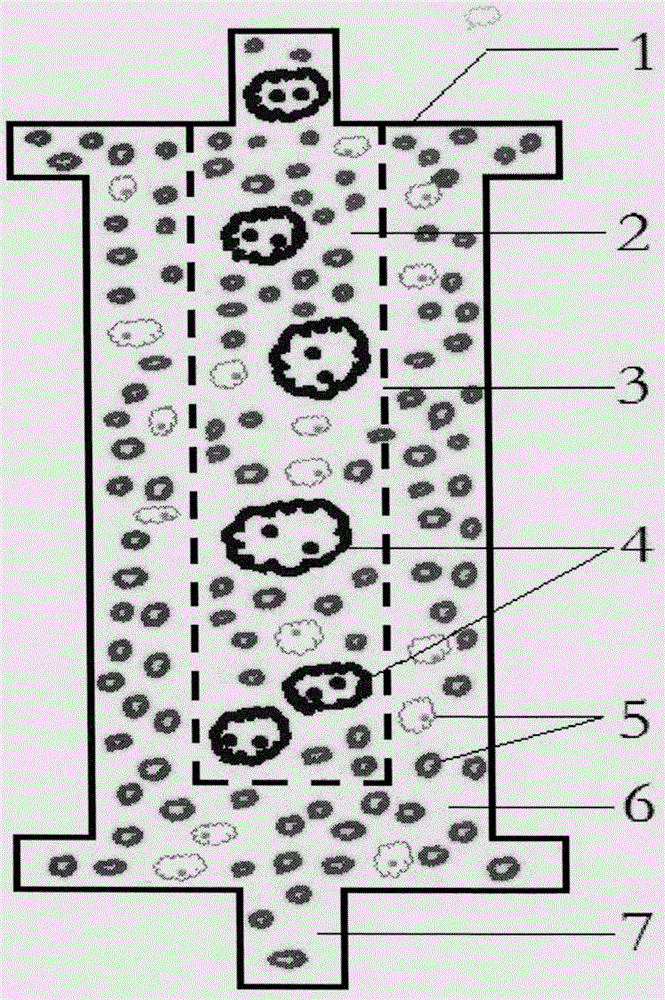
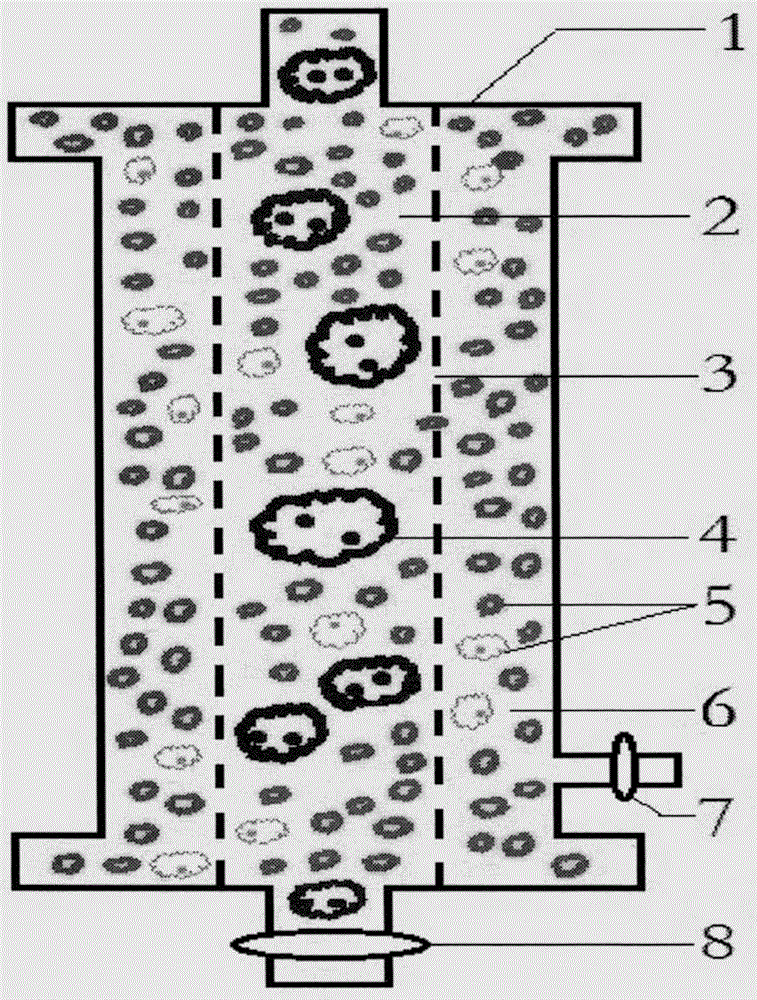
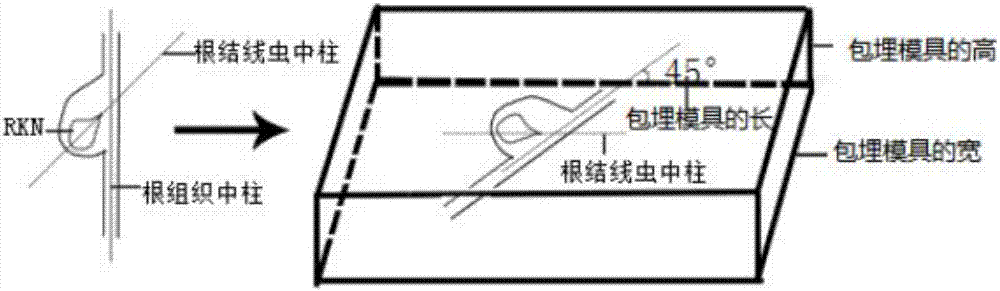
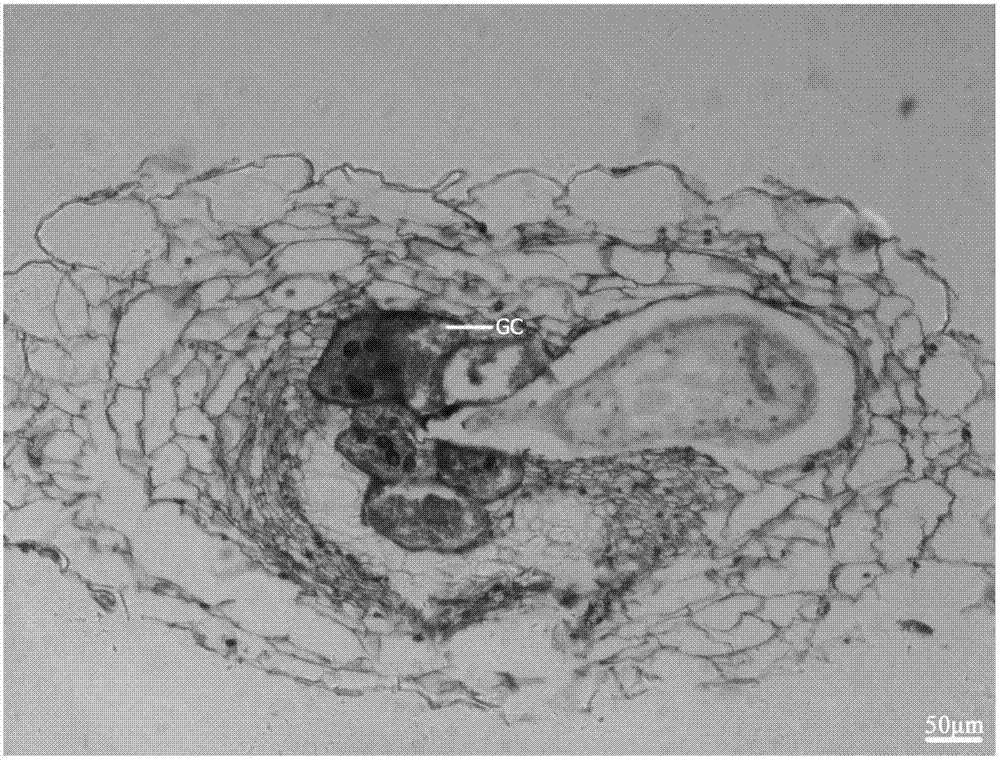
Method for effectively observing tomato root knot giant cell paraffin section
InactiveCN108007755ASlice Optimization ImprovementsQuality improvementInvestigation of vegetal materialPreparing sample for investigationParaffin sectionMultinucleate giant cell
The invention discloses a method for effectively observing a tomato root knot giant cell paraffin section. The method studies three key factors affecting the effect of the tomato root knot giant cellparaffin section, and the tomato root knot giant cell paraffin section technology includes a new material-taking and embedding method and a section selection technology is established. The technologyobviously improves the working efficiency of the tomato root knot giant cell paraffin section, improves the section selection quality, and ensures the effect of the tomato root knot giant cell paraffin section. The method has the advantages of high practicality and clear technical parameters, can be used for developing a related paraffin section embedding die and a matched paraffin section specialtechnology, and can be widely promoted and applied.
Owner:SOUTH CHINA AGRI UNIV
AIDS (Acquired Immune Deficiency Syndrome) blood purification therapeutic apparatus
ActiveCN106075626AAchieve high purificationEfficient purificationOther blood circulation devicesHaemofiltrationT cellBlood plasma
The invention discloses an AIDS (Acquired Immune Deficiency Syndrome) blood purification therapeutic apparatus, comprising a blood and blood serum separator capable of separating blood serum, single blood cell from multinucleated giant cells formed by inquilinous HIV (Human Immunodeficiency Virus) and comprising a purifier including an HIV antibody capable of being combined with the HIV, a CD4+T cell line and a hybridoma macrophage cell line. When blood flows through the separator, the multinucleated giant cells containing the HIV are filtered out; when the separated blood serum flows through the purifier, the HIV is adsorbed and cleaned by a purification agent; the purified blood serum and the single blood cell separated by the separator are converged and then are conveyed back to a body, so that the AIDS blood purification therapeutic aim of cleaning the HIV in and out of the blood cells is realized.
Owner:ZHEJIANG UNIV
Artificially synthesized cationic peptide and applications thereof in preparing anti-tumor drug
ActiveCN103992394ARelatively small molecular massImprove anti-tumor activityPeptide/protein ingredientsDepsipeptidesSide effectApoptosis
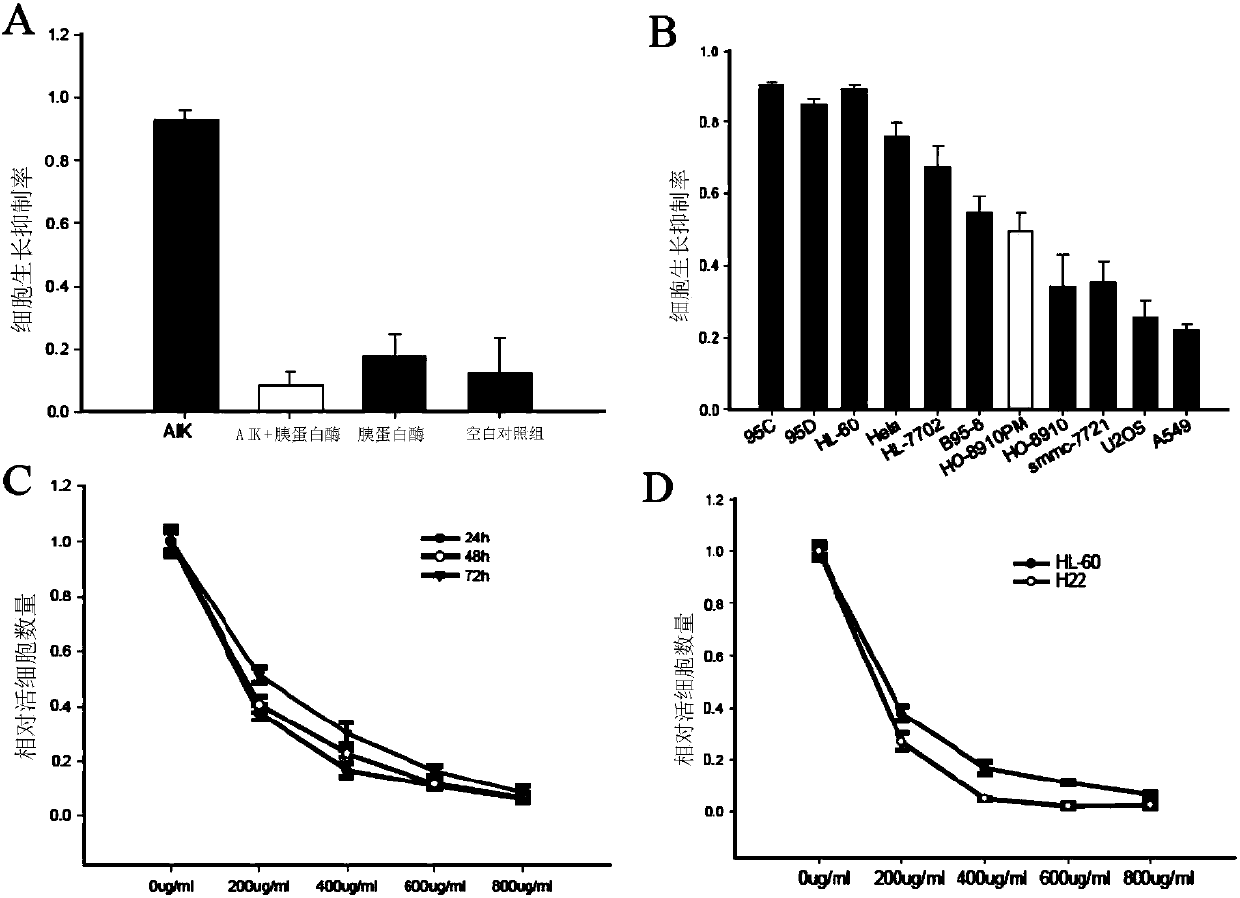
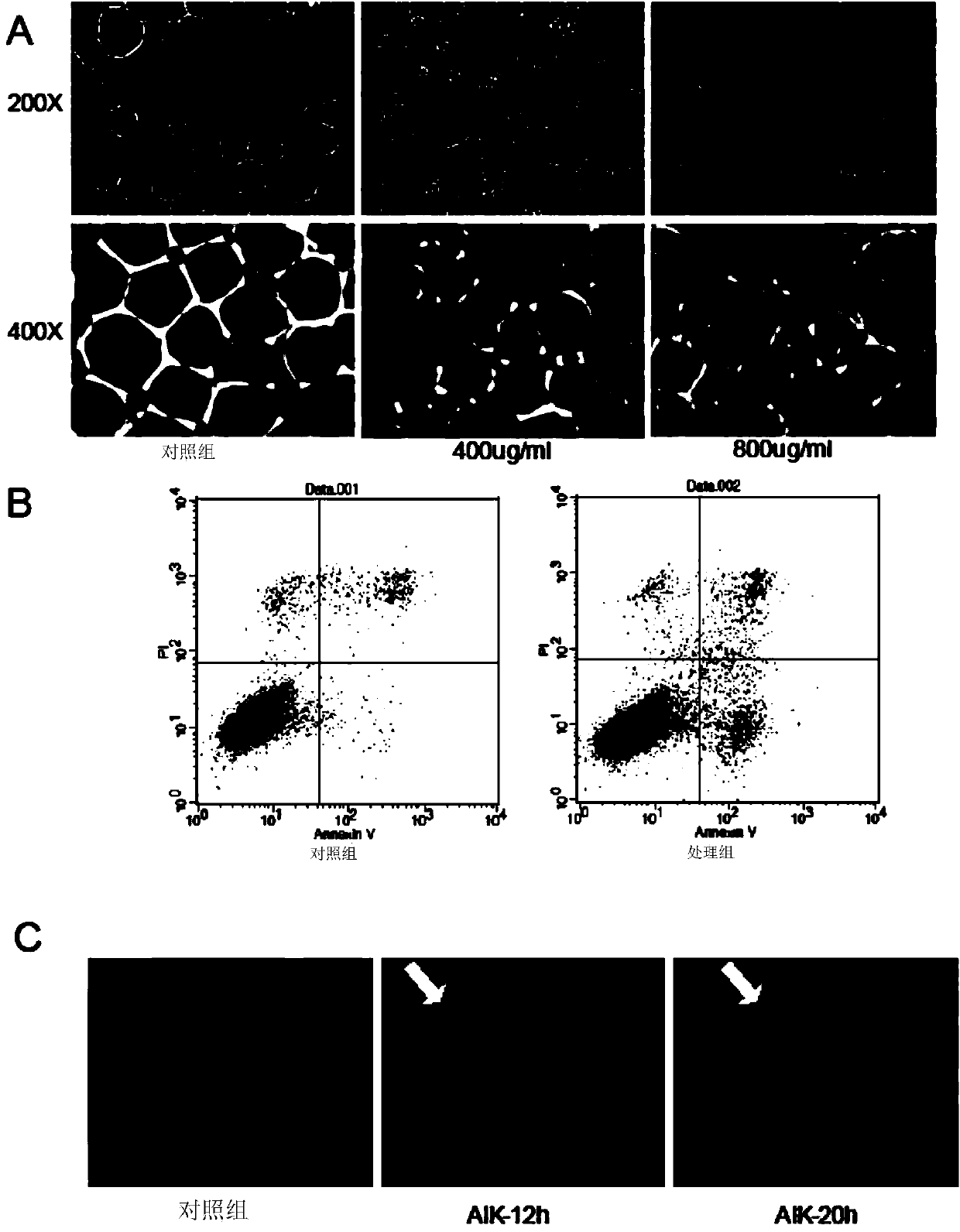
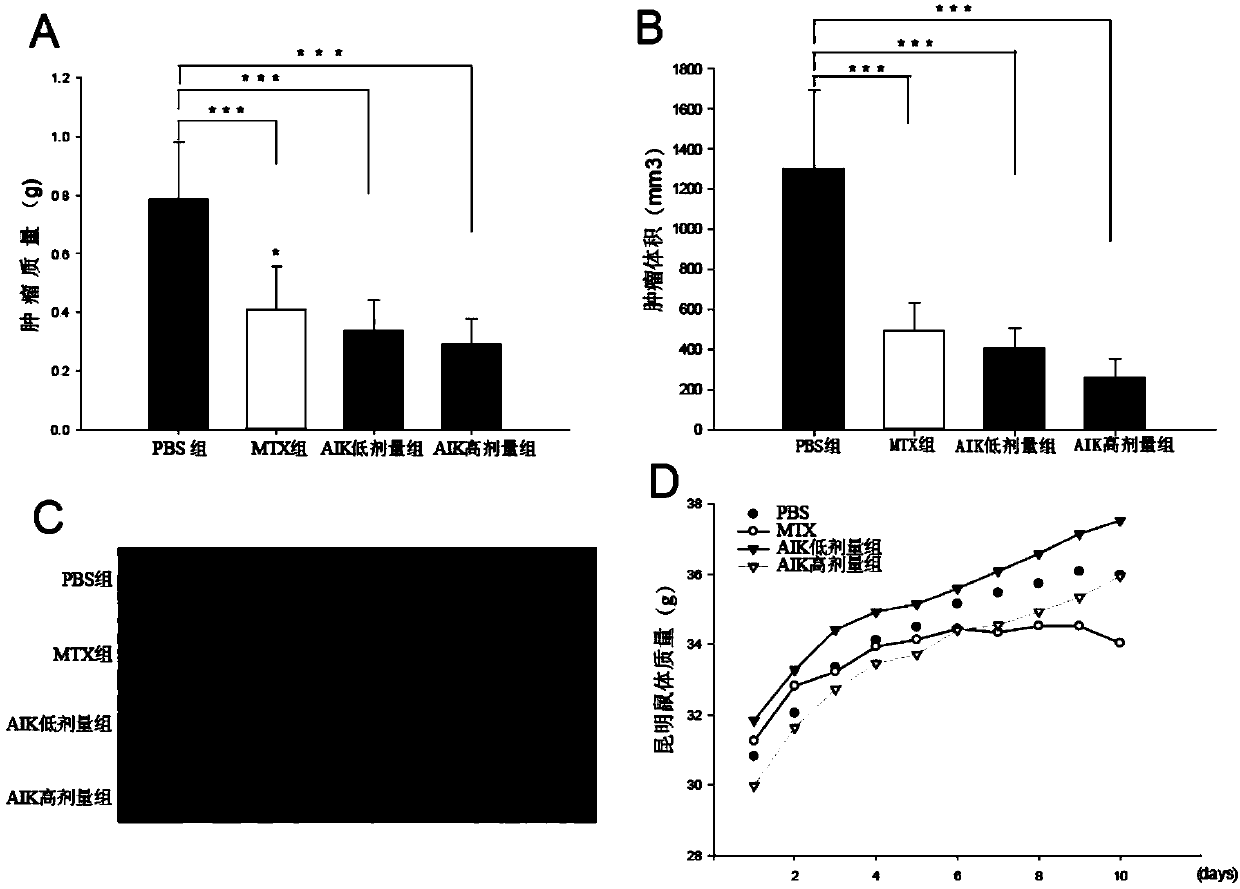
The invention discloses an artificially synthesized cationic peptide (named as AIK) and applications thereof in preparing an anti-tumor drug. The amino acid sequence of the cationic peptide is shown as SEQ ID NO.1. A research shows that the AIK has an obvious inhibition effect on various solid tumor cells, especially leukemic cells, cells of pulmonary giant cell cancer and hepatoma ascites cells, the anti-tumor activity is possibly combined an anionic acceptor on the surface of tumor cells so as to induce the tumor cells to be subjected to apoptosis and necrosis, and the anti-tumor activity is higher than that of the anti-tumor peptide molecules reported by other researchers. More importantly, the artificially synthesized 25-peptide drug molecules have higher specificity on the tumor cells. After the anti-tumor drug is locally administrated by subcutaneous tumor-bearing mice, the subcutaneous growth of liver cancer cells H22 of the tumor-bearing mice can be obviously inhibited and toxic and side effects are lower than chemotherapeutic drugs amethopterin. The artificially synthesized cationic peptide can provide theoretical basis and experimental materials for further researching and developing polypeptide anti-tumor new drugs and small-size targeted anti-tumor new drugs.
Owner:HARBIN MEDICAL UNIVERSITY
HIV (human immunodeficiency virus) immune purifier
ActiveCN106267412AIncrease concentrationReduce concentrationOther blood circulation devicesApparatus sterilizationBlood plasmaHuman immunodeficiency
An HIV (human immunodeficiency virus) immune purifier for the field of medical science is characterized in that a blood separator is prepared to filter multinuclear giant cells formed by lodging of HIV, a plasma separator is prepared to separate plasma and single blood cells, an HIV gp120 antibody and an HIVgp41 antibody capable of combining the HIV and HIV gp120 and gp41 antibodies capable of combining anti-goat Ig are prepared to serve as purifying agents distributed in agar gel, the agar gel is wrapped by high polymer materials to prepare the purifier, the purifier, the blood separator and the plasma separator jointly form critical components of an extracorporeal blood circulation device, the multinuclear giant cells containing the HIV are filtered when blood flows through the blood separator, the HIV is adsorbed and removed by the purifying agents when the plasma separated by the plasma separator flows through the purifier, and the purified plasma is converged with the single blood cells separated by the plasma separator and then returned into a body, so that HIV blood purification treatment of removing the HIV inside and outside the blood cells is achieved.
Owner:ATTACHED OBSTETRICS & GYNECOLOGY OSPITAL MEDICALCOLLEGE ZHEJIANG UNIV +1
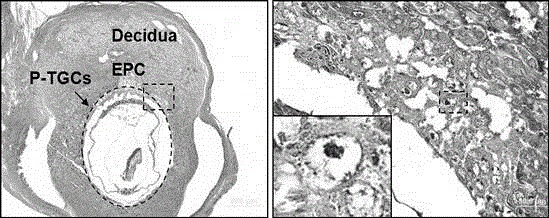
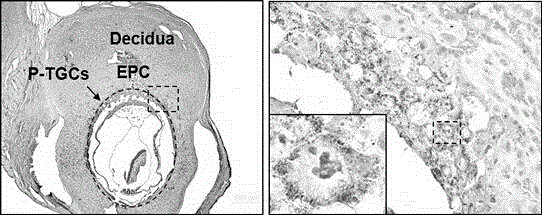
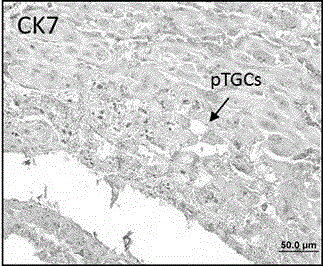
Application of p110 delta and antibody of p110 delta in specifically marking trophoblast giant cell (pTGC)
ActiveCN104155440AOvercome complex operation, time-consuming and other problemsAuxiliary indicator detectionMicrobiological testing/measurementDisease diagnosisIn situ hybridisationImmunofluorescence
The invention discloses an application of p110 delta and an antibody of the p110 delta in specifically marking an early trophoblast giant cell (Parietal TGC, pTGC). The p110 delta is a segment of p110 delta; an amino acid sequence of the segment is as described as SEQIDNO.1. According to a record, a p110 delta antibody is used as a detection medium for specifically marking pTGC by using conventional technical methods such as an immunohistochemical method, an immunofluorescence method or a polymerase chain reaction (PCR) method; a marker is relatively simple and convenient; the detection time is greatly shortened; the marker is easy to identify, relatively high in success rate, relatively good in user acceptance and relatively convenient to use in clinic. In addition, compared with those of the nucleic acid of hybridization in situ, the observation index is in the protein level; the organism effect condition can be reflected relatively directly; the application range is relatively wide.
Owner:GUANGDONG PHARMA UNIV
Aids immunotherapy instrument
ActiveCN106110426AFacilitate the binding reactionNot easy to fixOther blood circulation devicesT cellBlood plasma
The invention discloses an aids immunotherapy instrument for the medical field. The aids immunotherapy instrument is characterized in that a blood separator and a plasma separator capable of separating plasma, single hemocytes and multinuclear giant cells formed due to HIV parasitism are prepared, an HIVgp120 antibody and an HIVgp41 antibody capable of being bound with the HIV, an HIVgp120 antibody and a gp41 antibody capable of being bound with goat-anti Ig, and a CD4+T cell strain are prepared and blended into agar gel as a purifying agent, a purifier is made in a high polymer material wrapping mode, and the purifier and the separators together form key parts of an external blood circulation device. When blood flows through the blood separator, multinuclear giant cells containing the HIV are filtered out; when plasma separated by the plasma separator flows through the purifier, the HIV in plasma is adsorbed and removed by the purifying agent, purified plasma and single hemocytes separated by the plasma separator are mixed and then transfused back into the body, and thus the aids blood purification treatment purpose of removing the HIV inside and outside hemocytes is achieved.
Owner:翁炳焕 +1
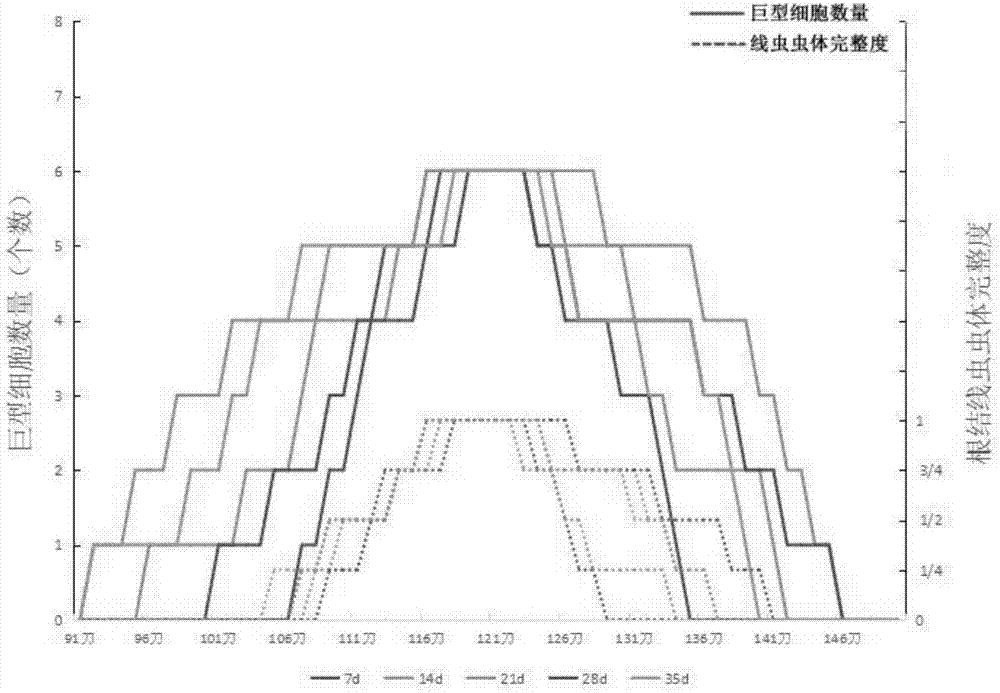
Method for identifying resistance of plant to meloidogyne
The invention discloses a method for identifying resistance of a plant to meloidogyne. The method comprises the steps of 1, grafting meloidogyne on a plant, and conducting cultivation; 2, after the step 1 finishes, taking plant root tissue, and observing the growth state of meloidogyne in the plant root tissue; 3, after the step 1 finishes, taking the plant root tissue, and calculating the number of meloidogyne in the plant root tissue; judging the resistance of the plant to meloidogyne according to the result. According to the method for identifying the resistance of the plant to meloidogyne, toluidine blue is applied to dyeing of a root-knot paraffin slice, an optimal dyeing time is found, and the growth state of the nematodes and giant cells can be clearly observed; moreover, the dyeing time is fast, and the effect is good. Aiming at the statistics on the number of the nematodes, a grinding method and a screening method are combined, an optimal grinding time is found, and convenience is brought to the statistics on the number of the nematodes. The two methods can combined into application, and the resistance of the plant to the statistics on the number of the nematodes can be expressed from two aspects of the growth state and the nematodes and the number of the nematodes.
Owner:BEIJING UNIV OF AGRI
AIDS blood plasma purifying therapeutic instrument
An AIDS blood plasma purifying therapeutic instrument in the medical science field comprises a blood separator, a blood plasma separator and a blood plasma purifier; the blood plasma separator can separate blood plasma from single blood corpuscles; the blood separator can filter multinuclear giant cells formed by lodging HIV; the blood plasma purifier can prepare and amplify infinite growing hybridomas macrophage strains with phagocytosis characteristic, the hybridomas macrophage strains are mixed in a cell frozen stock solution so as to enable cell concentration to reach 80%, and the purifier is made by pouring high-molecular material; the macrophage strains can swallow HIV; the purifier, the blood separator and the blood plasma separator can form a key external cycle system; when flood flows to pass the blood separator, the multinuclear giant cells containing HIV can be filtered; when the blood plasma separated by the blood plasma separator passes the purifier, containing HIV can be removed by a cleanser; the purified blood plasma can gather with single blood cells separated by the blood plasma separator, and can be returned, thus realizing purifying treatment purpose by removing HIV inside and outside blood cells.
Owner:树兰(杭州)医院有限公司
Traditional Chinese medicine for treating haemorrhoids and preparation method thereof
InactiveCN107456478APrevent proliferationEffective in treating hemorrhoidsAntineoplastic agentsMagnoliophyta medical ingredientsDesmodium microphyllumA549 cell
The invention discloses a traditional Chinese medicine for treating haemorrhoids. The traditional Chinese medicine is prepared from the following raw materials in parts by weight: 25-35 parts of delavay pararuellia herb, 10-20 parts of desmodium microphyllum, 10-18 parts of chicken's-claw wind and 8-12 parts of hairyfruit croton leaves and roots. A corresponding preparation method is adopted. The traditional Chinese medicine provided by the invention has the functions of treating haemorrhoids and inhibiting cell proliferation of human giant cell lung cancer cells A549.
Owner:NANJING ZHENGKUAN MEDICAL TECH
AIDS biological cell therapeutic instrument
ActiveCN106267404AFacilitate the binding reactionEasy to retainOther blood circulation devicesHaemofiltrationExtracorporeal circulationRed blood cell
The invention provides an AIDS biological cell therapeutic instrument in the field of medicine. The AIDS biological cell therapeutic instrument is characterized in that blood and plasma separators capable of separating plasma, single blood cell and multinucleated giant cells formed by the lodging of HIV is prepared, a hybridoma cell strain both keeping the phagocytic characteristics and performing infinite growth is prepared, the hybridoma cell strain is wrapped with a high polymer material to prepare a purifier after being amplified, then agarose having a gradient concentration is added to fix the hybridoma cell strain to swallow HIV, the purifier constitutes a critical extracorporeal circulation device with the separators together, when the blood flows by the blood separator, the multinucleated giant cells containing the HIV are filtered, when the plasma separated by the plasma separator flows by the purifier, the HIV therein is cleared by the cleaning agent, the purified plasma is conveyed back in vivo after being combined with a single blood cell separated by the plasma separator, and thus the purification treatment purpose of clearing the HIV inside and outside the blood cells is realized.
Owner:运城同创医疗管理有限公司
AIDS biological cell immunotherapy instrument
The invention discloses an AIDS biological cellular immunotherapy apparatus, and belongs to the field of medicine. The AIDS biological cellular immunotherapy apparatus is characterized in that blood and plasma separators, by which plasma, individual blood cells and multinuclear giant cells formed due to HIV parasitism are prepared; SV40LTag-pcDNA3.1 (-) and pLXSNneo-hTERT recons are constructed; combined transfection is conducted, so as to cause macrophage immortalization; the macrophage, after being subjected to amplification, is prepared in a cell cryopreservation solution until a cell concentration is 80%, and then, a purifier, which is made from a high polymer material, is poured with cells, wherein macrophage cell lines take an effect on phagocytizing HIV; the purifier and the blood and plasma separators constitute a key extracorporeal circulation device; when blood passes through the blood separator, the multinuclear giant cells which contain the HIV are filtered and removed, and subsequently, when plasma, which is separated by virtue of the plasma separator, passes through the purifier, the HIV in the plasma is phagocytized by the macrophage; and the purified plasma is mixed with the individual blood cells separated by virtue of the plasma separator, and then, the mixed plasma and individual blood cells are conveyed back, so that the purification treatment purpose of clearing the HIV inside and outside the blood cells is achieved.
Owner:ATTACHED OBSTETRICS & GYNECOLOGY OSPITAL MEDICALCOLLEGE ZHEJIANG UNIV +1
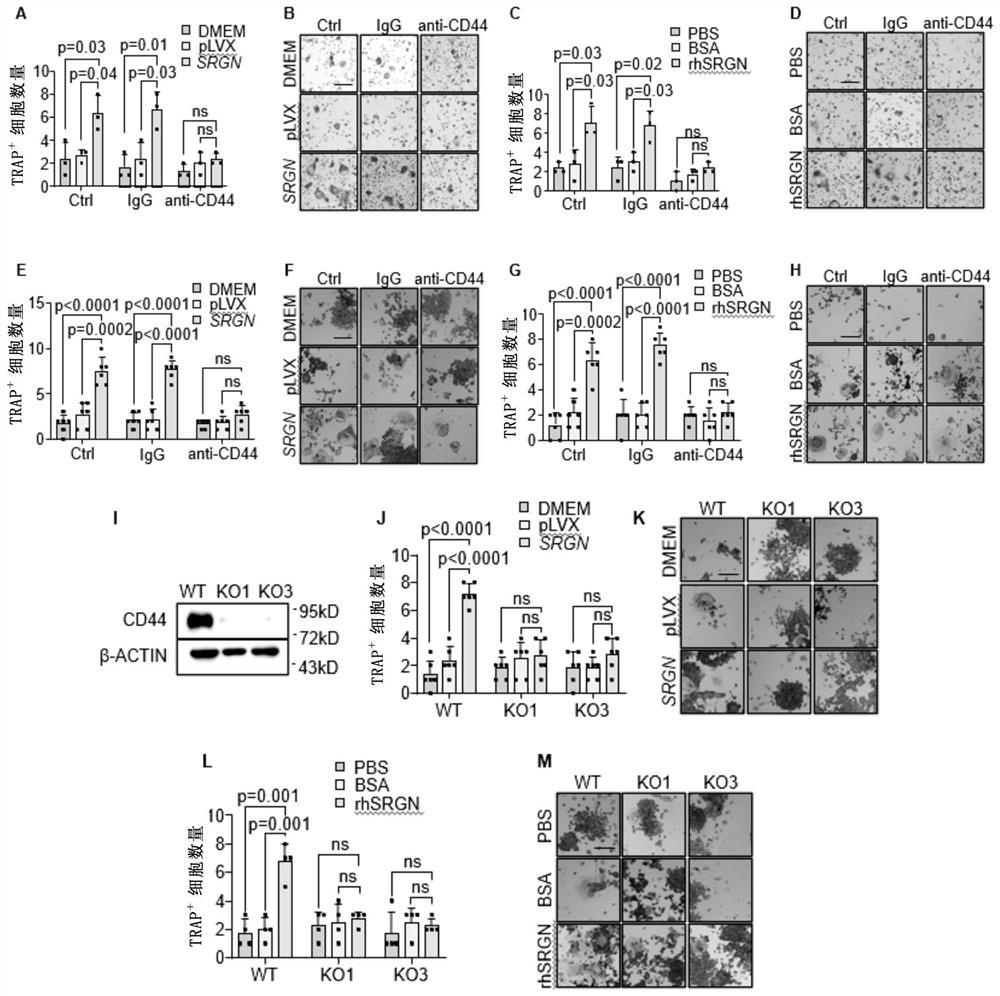
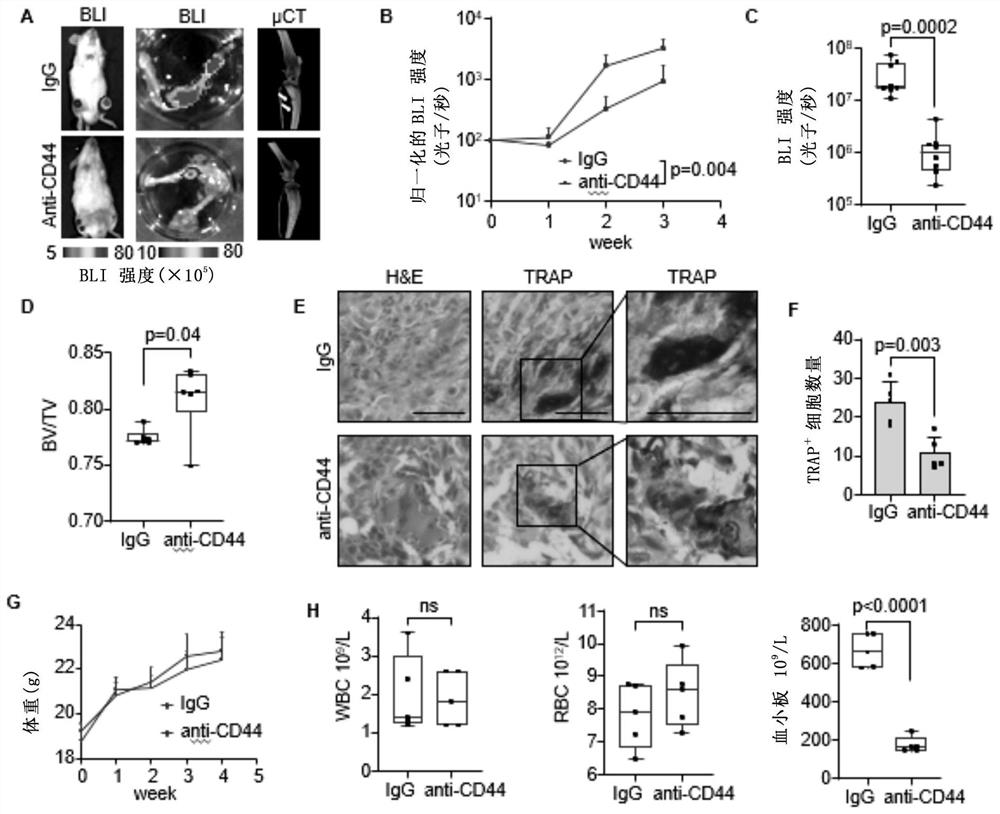
Method for treating giant cell tumor of bone
The invention relates to a method for treating giant cell tumor of bone. Specifically, the invention provides application of a CD44 inhibitor, and the CD44 inhibitor is used for preparing the medicine for treating the giant cell tumor of the bone. Experimental results of the invention show that, the SRGN can be used as a novel biological marker for predicting the giant cell tumor of the bone and is used for diagnosing, typing and / or treating the giant cell tumor of the bone. Besides, in the tissue of giant cell tumor of the bone, the fusiform stromal cells can secrete glycoprotein of SRGN and can interact with the CD44 receptor on the mononuclear cells, so that the mononuclear cells are promoted to be differentiated into multinuclear giant cells. Therefore, the CD44 can be used as a target for clinically treating the giant cell tumor of the bone.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Human cytomegalo virus immunogen fusion protein as well as preparation method and usage thereof
ActiveCN102816246BImproving immunogenicityGood immune effectViral antigen ingredientsAntibody mimetics/scaffoldsDiseaseEukaryotic plasmids
The invention discloses a human cytomegalovirus immunogen fusion protein, which includes flagellin, and human cytomegalovirus envelope glycoprotein or its polypeptide fragment; the invention discloses a DNA molecule encoding the aforementioned fusion protein, and a DNA molecule comprising the DNA Molecular recombinant plasmid and recombinant expression vector; the invention discloses the preparation method and application of the fusion protein; the invention also discloses a human cytomegalovirus vaccine. The fusion protein of the invention has strong immunogenicity, can stimulate the body to produce a large amount of anti-cytomegalovirus antibodies, is used for preparing vaccines to prevent diseases caused by cytomegalovirus infection, and has good clinical application prospects.
Owner:CHENGDU RONGSHENG PHARMA
Non-tumorigenic mdck cell line and screening method for amplifying influenza virus
ActiveCN104059873BReduced tumorigenicityIncreased tumorigenicityMicroorganism based processesVertebrate cellsScreening methodCell growth rate
The invention discloses a non-tumor-causing MDCK cell line for amplifying influenza virus and a screening method thereof. The cell line is MDCK‑2B2, and the preservation number is CCTCC C201323. The present invention provides a non-tumorigenic cell line MDCK-2B2 and its subclone cell line, which grow into adherent cells and / or have epithelial-like morphology and / or can support the replication of various viruses, and can amplify Higher titer virus; In addition, the present invention has found a method for screening non-tumorigenic cell lines, which can assist the judgment of tumorigenicity through the growth rate of cells and the morphological changes of cells, that is, relatively slow-growing single-pore cells, which The tumorigenicity will be significantly reduced, and the appearance of giant cells will significantly increase the tumorigenicity. When screening non-tumorigenic cell lines on a large scale, such auxiliary judgment methods can shorten time and save costs.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
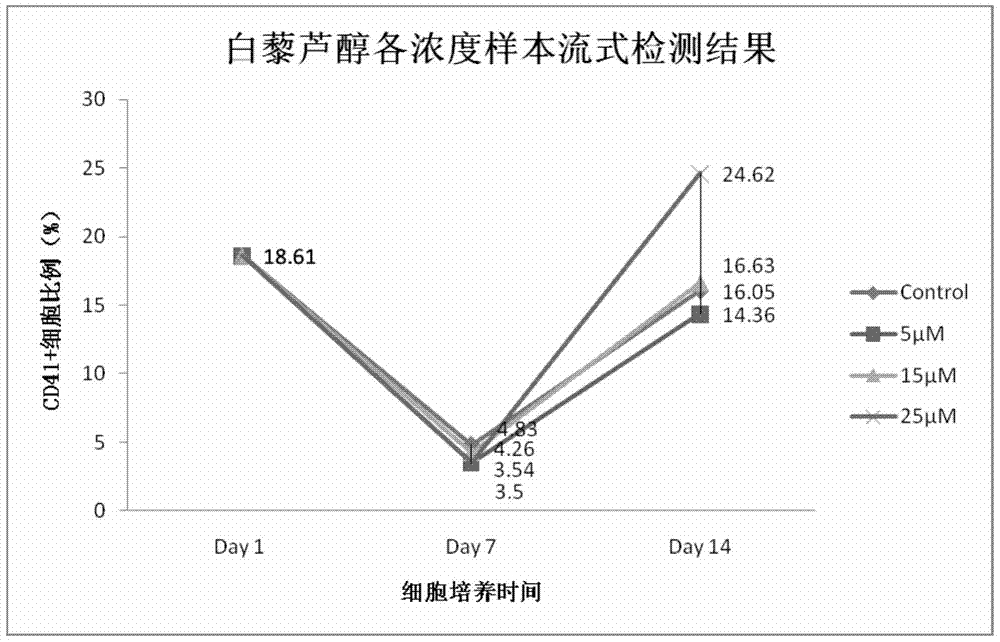
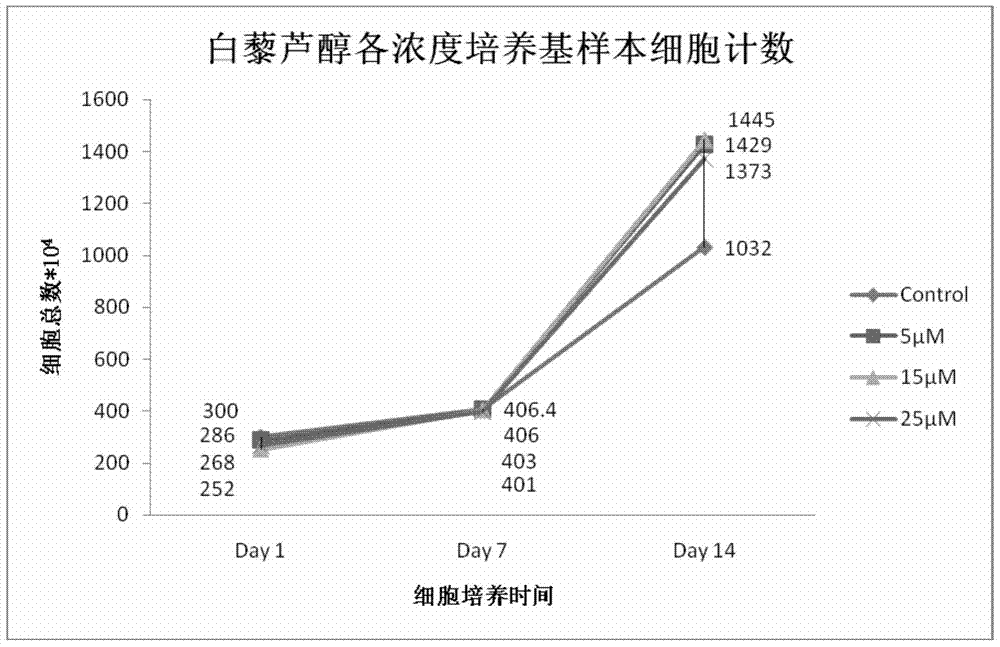
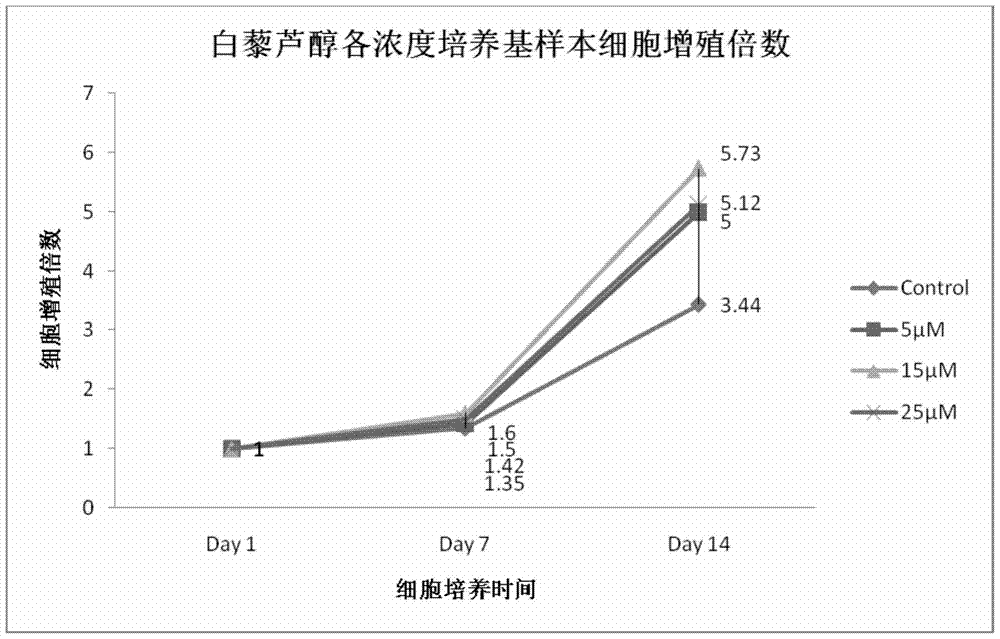
A method for increasing the number of directional differentiation of umbilical cord blood megakaryotic progenitor cells
ActiveCN104789530BIncrease the number ofLow costBlood/immune system cellsProgenitorCord blood stem cell
The invention discloses a method for increasing the number of directional differentiation of umbilical cord blood megakaryocyte progenitor cells. In this method, umbilical cord blood hematopoietic stem cells are used as sample cells, and directed differentiation is carried out in a specific medium containing resveratrol. The specific medium containing resveratrol makes the operation of directed differentiation of megakaryotic progenitor cells easier, safer and more efficient. In the process of directional differentiation and culture of umbilical cord blood hematopoietic stem cells to megakaryotic progenitor cells, resveratrol is added together with cytokines to achieve the effect of increasing the number of megakaryotic progenitor cells. The method of the present invention is easy to operate and low in cost. Cell safety is high.
Owner:广州市天河诺亚生物工程有限公司
AIDS detox treatment instrument
ActiveCN106267411BIncrease concentrationLow concentration of agaroseOther blood circulation devicesRed blood cellBlood plasma
An AIDS detoxification treatment instrument used in the medical field, characterized in that it prepares a blood and plasma separator capable of separating plasma, single blood cells and multinucleated giant cells formed by inhabiting HIV, and prepares HIVgp120 antibodies and HIVgp41 antibodies that can bind HIV As well as HIV gp120 and gp41 antibodies combined with goat anti-Ig, and hybridoma macrophage cell lines, as purifiers, prepared in agar gel, wrapped with polymer materials to make a purifier, and together with the separator constitute the key to the extracorporeal blood circulation device When the blood flows through the blood separator, the multinucleated giant cells containing HIV are filtered out, and then when the plasma separated by the plasma separator flows through the purifier, the HIV in it is absorbed and removed by the purifier, and the purified plasma and plasma The single blood cells separated by the separator are confluent and then reinfused into the body, so as to achieve the purpose of AIDS blood purification treatment to remove HIV inside and outside the blood cells.
Owner:翁炳焕 +1
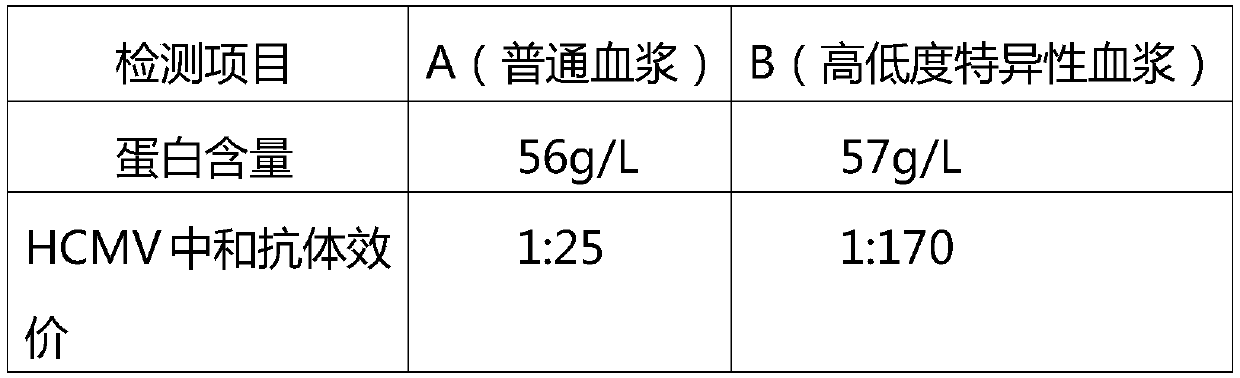
A kind of intravenous giant cell human immunoglobulin and its preparation method
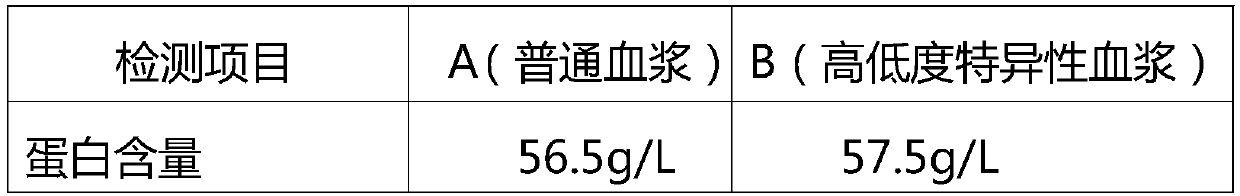
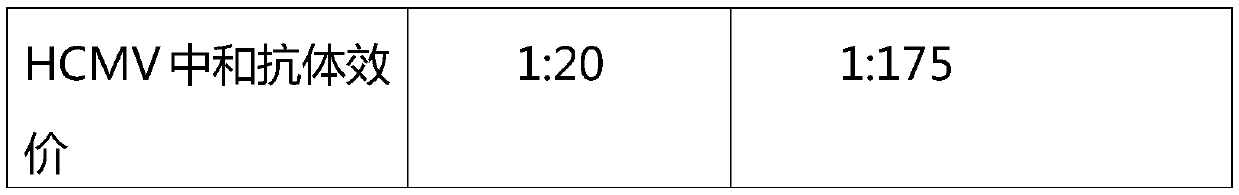
ActiveCN105601735BImprove survival rateStrong specificityImmunoglobulins against virusesPeptide preparation methodsFiltrationUltrafiltration
The invention relates to intravenously injected cytomegalovirus human immune globulin and a preparation method thereof, and the titer of a cytomegalovirus neutralizing antibody of the human immune globulin is larger than or equal to 1:500. The preparation method comprises the steps that 1, positive plasma with the titer of the cytomegalovirus neutralizing antibody larger than or equal to 1:20 is screened out; 2, the screened-out efficient positive plasma is mixed; 3, the mixed plasma is separated through a low-temperature ethanol filter-pressing method, an immune globulin component II is separated and purified by combining an ion-exchange column chromatography method, viruses are removed through filtration, chromatography, ultrafiltration, preparation, low-pH incubation virus inactivation and nanofilm filtration, the immune globulin with the purity of 98.5%-100% is obtained through subpackaging, and the antibody titer is not lower than 1:500. The cytomegalovirus human immune globulin prepared through the preparation and production method is high in antibody titer, purity and recovery rate and capable of conducting targeted treatment on cytomegalovirus, is an effectively drug for treating recessive and dominant infection caused by the cytomegalovirus in people, is safe and reliable and has larger social benefits and economic benefits.
Owner:哈尔滨派斯菲科生物制药有限公司
AIDS Infected Cell Separator
ActiveCN106267410BTo achieve the therapeutic purpose of immune reconstructionOther blood circulation devicesInfected cellCell separation
The invention relates to an AIDS-infected cell separator in the field of biomedicine, which is characterized in that it is made of a high-biocompatibility material that can pass through a single blood cell and chemical components but cannot pass through two or more large-volume cells that are bonded together. 150~250μm, 50~150μm, 15~40μm, 8~15μm, 5~8μm, 3~5μm, 1~2μm, correspondingly calibrated as type I~VII cell separators, simultaneously prepare HIVgp120 and CD4 antibodies, HIV infection The gp120 on the cell surface can bind other CD4+ cells by itself or under the action of gp120 antibody or CD4 antibody to form large multinucleated giant cells. Type I-II separators are used to separate super-large fungi, spirochetes, and tumor cells. HIV-infected large-volume multinucleated giant cells, multicellular aggregates, CD4+ cells susceptible to HIV infection, and AIDS-infected cells are separated and cleared in the extracorporeal circulation composed of separators and other components.
Owner:翁炳焕 +1
A method for identifying resistance of plants to root-knot nematodes
Owner:BEIJING UNIV OF AGRI
aids plasma purification treatment instrument
An AIDS blood plasma purifying therapeutic instrument in the medical science field comprises a blood separator, a blood plasma separator and a blood plasma purifier; the blood plasma separator can separate blood plasma from single blood corpuscles; the blood separator can filter multinuclear giant cells formed by lodging HIV; the blood plasma purifier can prepare and amplify infinite growing hybridomas macrophage strains with phagocytosis characteristic, the hybridomas macrophage strains are mixed in a cell frozen stock solution so as to enable cell concentration to reach 80%, and the purifier is made by pouring high-molecular material; the macrophage strains can swallow HIV; the purifier, the blood separator and the blood plasma separator can form a key external cycle system; when flood flows to pass the blood separator, the multinuclear giant cells containing HIV can be filtered; when the blood plasma separated by the blood plasma separator passes the purifier, containing HIV can be removed by a cleanser; the purified blood plasma can gather with single blood cells separated by the blood plasma separator, and can be returned, thus realizing purifying treatment purpose by removing HIV inside and outside blood cells.
Owner:树兰(杭州)医院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com