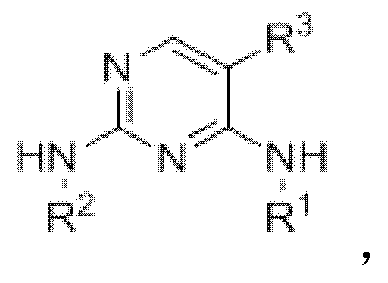
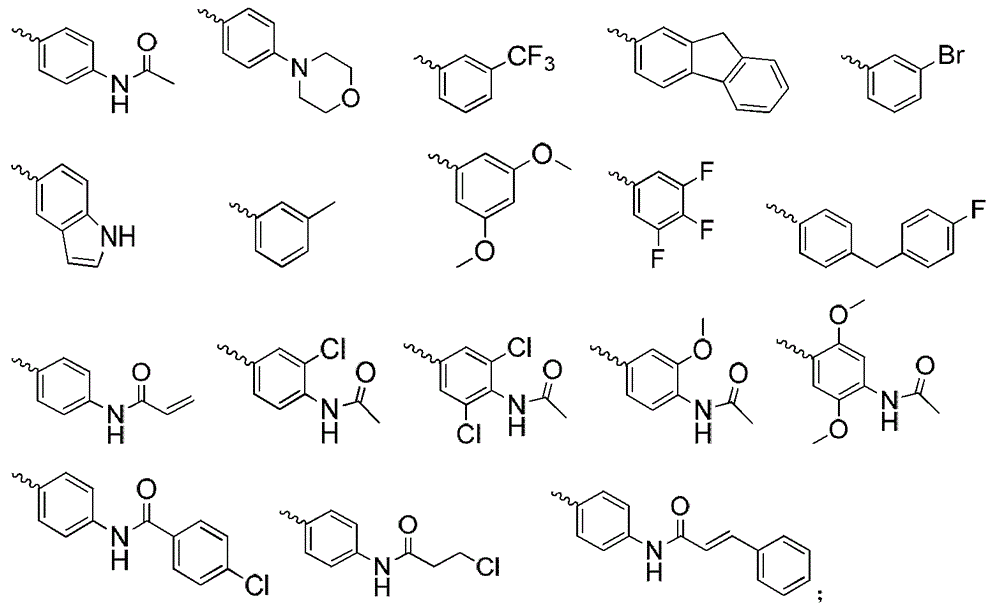
2,4,5-trisubstituted pyrimidine compounds taking FGFRs (fibroblast growth factor receptors) as targets as well as preparation methods and application of 2,4,5-trisubstituted pyrimidine compounds
A tri-substituted, pyrimidine technology, applied in the field of medicinal chemistry, to achieve the effect of good anti-tumor effect and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of N-{4-{{2-{[4-methylpiperazin-1-yl]phenyl}amine}-5-nitropyrimidin-4-yl}benzene}acetamide (compound 1)
[0037]
[0038] (1) Preparation of N-{4-[(2-chloro-5-nitropyrimidin-4-yl)amine]phenyl}acetamide
[0039]
[0040] Under the condition of ice bath, 2,4-dichloro-5-nitropyrimidine (5g, 25.7mmol) was dissolved in dichloromethane (100ml), and N,N-diisopropylethylamine (4.3ml , 25.7mmol) and acetaminoaniline (3.87g, 25.7mmol). The ice bath was removed, and the reaction mixture was reacted at room temperature for 5 h, and then saturated brine (200 ml) was added. The mixture was extracted with dichloromethane (300ml). The organic layer was dried with anhydrous magnesium sulfate and concentrated to obtain a solid. The product was not further purified to obtain 6.34 g of a red solid with a yield of 90.0%.
[0041] (2) Preparation of N-{4-{{2-{[4-methylpiperazin-1-yl]phenyl}amine}-5-nitropyrimidin-4-yl}benzene}acetamide (compound 1)
[0042] ...
Embodiment 2
[0044] Example 2 N-{4-{{5-chloro-2-{[4-(4-methylpiperazin-1-yl)phenyl]amino}pyrimidin-4-yl}amine}phenyl}acetamide (Compound 2) Preparation
[0045]
[0046] (1) Preparation of N-{4-[(2,5-dichloropyrimidin-4-yl)amine]phenyl}acetamide
[0047] Weigh paracetamol (1.31g, 8.72mmol) and dissolve it in DMF (20ml), then add anhydrous potassium carbonate (2.42g, 17.5mmol) and 2,4,5-trichloropyrimidine (1.0ml, 8.72mmol) . The reaction mixture was heated to 60°C for 2h. After the reaction was completed, it was cooled to room temperature, and a large amount of ice water was added to precipitate a solid, which was filtered by suction and dried. The product was not further purified, and 2.3 g was obtained with a yield of 90%.
[0048] (2) N-{4-{{5-chloro-2-{[4-(4-methylpiperazin-1-yl)phenyl]amino}pyrimidin-4-yl}amine}phenyl}acetamide preparation of
[0049] Weigh N-{4-[(2,5-dichloropyrimidin-4-yl)amine]phenyl}acetamide (1.6g, 5.45mmol), 4-methylpiperazine aniline (1.24g, 6.46mmol) ...
Embodiment 3
[0055] Example 3 Determination of Compounds on FGFR Kinase Activity
[0056] The inhibitory activity of the compound on FGFR1 was determined by the LANCE ULTRA Assay method, and compared with the positive control drug, the compound with good activity was screened out. FGFR1 was purchased from CARNA Corporation.
[0057] Specific method: the tested compound, ATP, specific substrate and FGFR1 kinase are diluted with kinase diluent. Kinase reaction mixture contains FGFR1, ATP, substrate, HEPES (PH=7.5), MgCl 2 , EGTA, Tween-20. The group without any compound was used as the 100% phosphorylation control, and the EDTA termination reaction group immediately after adding FGFR1 kinase was used as the 0% phosphorylation control. After co-incubating the kinase reaction mixture for 1 h at room temperature, EDTA was added to stop the reaction for 5 min. Add the specific antibody again, continue co-incubating at room temperature for 1 h, and detect the excitation light with a PerkinElm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com