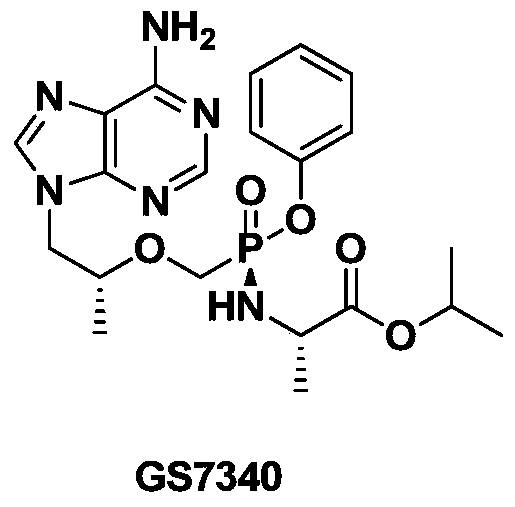
Nucleotide phosphonate compound, its pharmaceutical composition, preparation method and use
A compound and pharmaceutical technology, applied in the field of nucleotide phosphonate compounds, can solve the problems of unstable chemical properties, inability to effectively increase the concentration of drugs at the action site, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
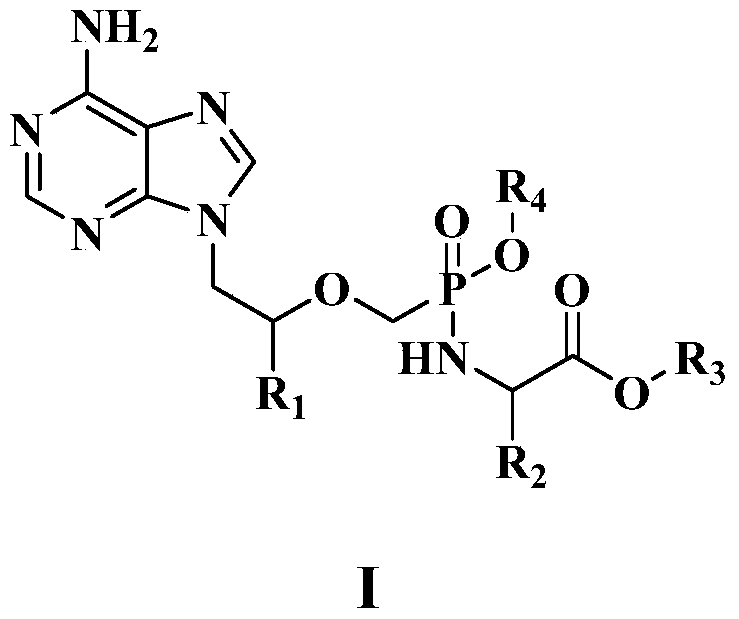
[0099] Example 1: 9-{(R)-2-[[(S)[[(S)-1-(isopropoxycarbonyl)ethyl]amino]-2,3-dihydro-1H-indene 5-oxyphosphonic acid]methoxy]propyl}adenine (compound 1a); and
[0100] 9-{(R)-2-[[(R)[[(S)-1-(isopropoxycarbonyl)ethyl]amino]-2,3-dihydro-1H-indenyl-5-oxy Of phosphonic acid]methoxy]propyl}adenine (compound 1b)
[0101]
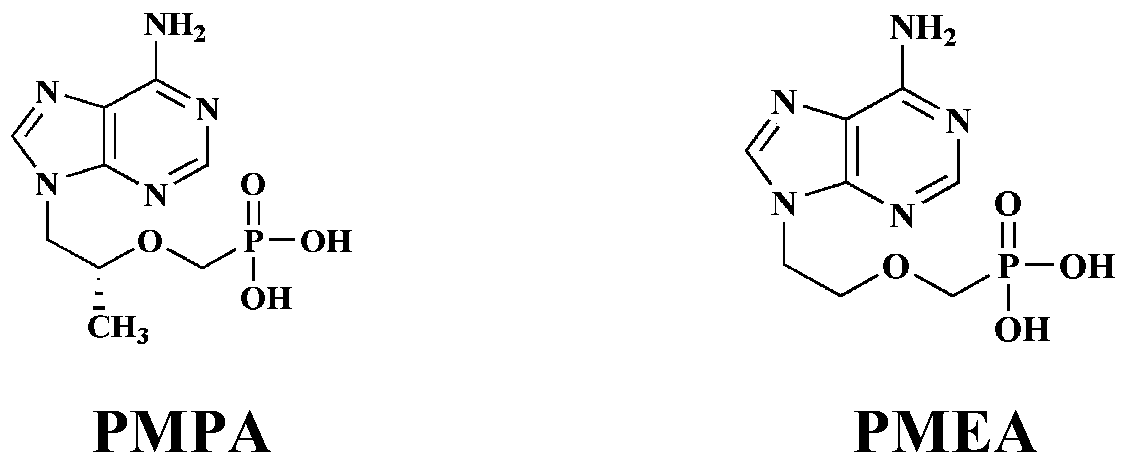
[0102] N 2 Under protection, 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) (287mg, 1.0mmol) was suspended in dichloromethane (20mL), and N, N- Diethylformamide (0.121mL, 1.1mmol), oxalyl chloride (0.31mL, 3.5mmol) were refluxed for 3h, cooled to room temperature, concentrated under reduced pressure, then dichloromethane (15mL) was added, and then concentrated under reduced pressure once, and two Chloromethane (15mL), cool to -20°C, add diisopropylethylamine (0.35mL, 2.0mmol) to make A solution for use.
[0103] Dissolve L-alanine isopropyl ester hydrochloride (167mg, 10mmol) and N,N-diisopropylethylamine (0.71mL, 4.0mmol) in dichloromethane (10mL) and stir at room ...
Embodiment 2
[0108] Example 2: 9-{(R)-2-[[[[((S)-1-(methoxycarbonyl)2-methylpropyl]amino]-2,3-dihydro-1H- Preparation of indenyl-5-oxyphosphonic acid]methoxy]propyl}adenine (compound 2)
[0109]
[0110] According to the operation of Example 1, with 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) (287mg, 1.0mmol), 5-indanol (134mg, 1.0mmol) and L-valine methyl ester hydrochloride (167mg, 1.0mmol) was used as the raw material to react to produce compound 2 (185mg, 36%).
[0111] 1 H-NMR (600MHz, CDCl 3 ,δ ppm ): 8.14(d,1H), 8.10(d,1H), 6.96(m,3H), 6.62(s,2H), 5.39(m,1H), 4.26(m,1H), 4.16(m,1H) ,3.94(m,1H), 3.83(m,2H), 3.65(m,1H), 3.55(d,3H), 2.80(m,4H), 2.00(m,2H), 1.04(m,3H), 0.79 (m, 6H). ES-API(m / z):[M+H] + 517.2.
Embodiment 3
[0112] Example 3: 9-{(R)-2-[[[[((S)-1-(methoxycarbonyl)ethyl]amino]-2,3-dihydro-1H-indenyl-5- Preparation of oxyphosphonic acid]methoxy]propyl}adenine (compound 3)
[0113]
[0114] According to the operation of Example 1, with 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) (287mg, 1.0mmol), 5-indanol (134mg, 1.0mmol) and L-alanine methyl ester hydrochloride (139mg, 1.0mmol) was used as a raw material to react to produce compound 3 (156mg, 32.1%).
[0115] 1 H-NMR (600MHz, CDCl 3 ,δ ppm ): 1 H-NMR (600MHz, CDCl 3 ,δ ppm ): 8.16(d,1H), 8.10(d,1H), 7.01(m,3H), 6.60(s,2H), 4.46(m,1H), 4.20(m,1H), 3.98(m,3H) , 3.71 (d, 3H), 3.65 (m, 1H), 2.82 (m, 4H), 2.01 (m, 2H), 1.27 (m, 6H). ES-API(m / z):[M+H] + 489.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com