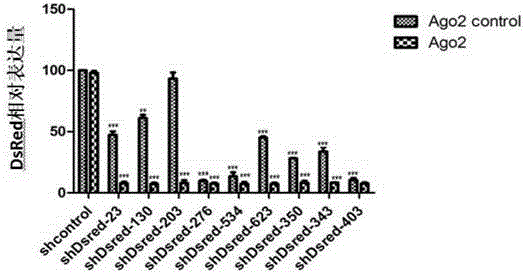
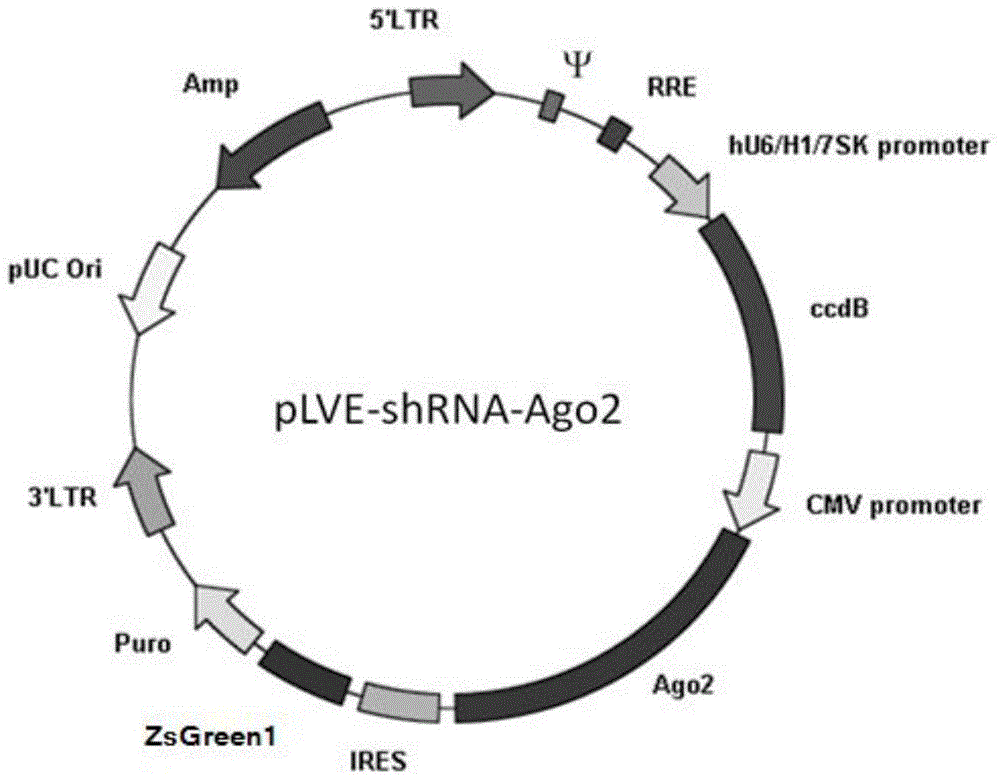
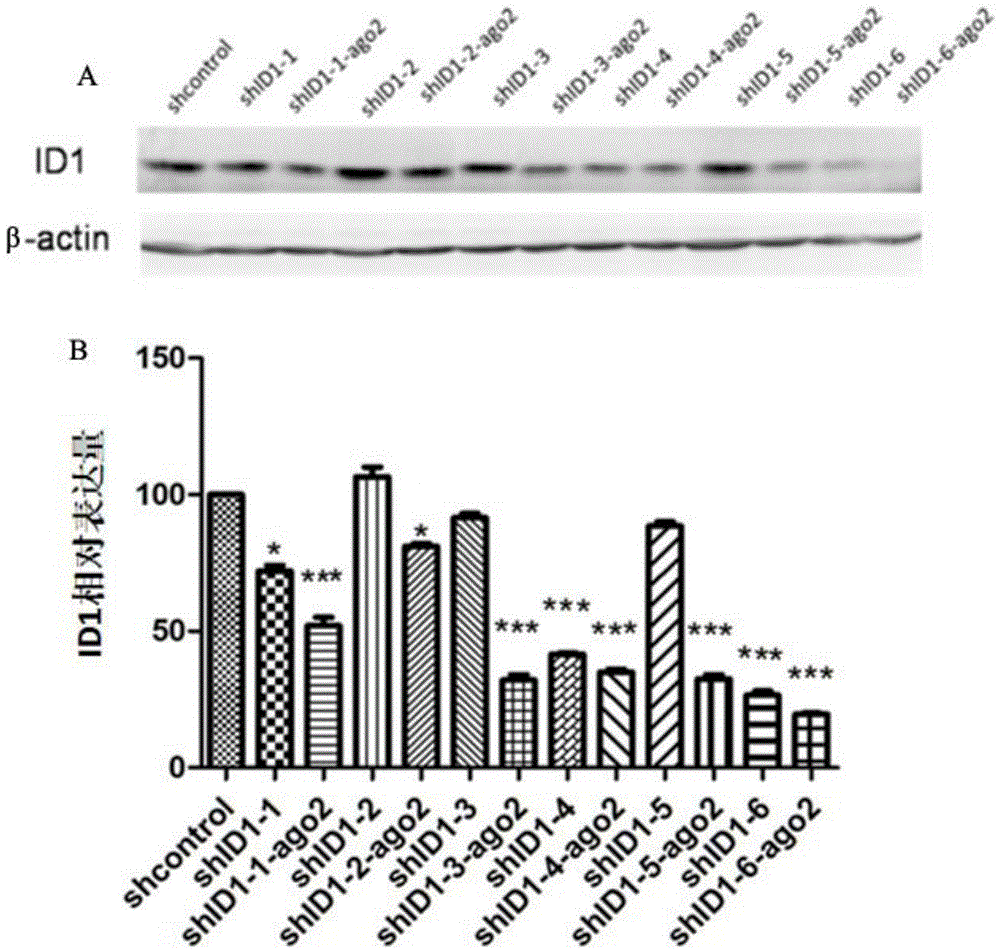
ShRNA-Ago2 coexpression lentivirus RNAi vector, recombinant plasmid and constructing method of recombinant plasmid
A recombinant plasmid, cmv-ago2 technology, applied in the field of lentiviral RNAi vectors, can solve the problems of low transfection efficiency, affecting the high-level expression of shRNA, hindering the interference effect of shRNA, etc., achieving the effect of high efficiency and simple and efficient construction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Construction of shRNA-Ago2 co-expression lentiviral RNAi vector
[0048] (1) Prepare the backbone pLVX-IRES-ZsGreen1-Puro for constructing the vector:
[0049] Using the pLVX-IRES-ZsGreen1 (Clontech) vector as a template, use the upstream primer (SEQ ID No: 1) TTTATCGATGGATCCTAACGCGTGAATTCGCCCCTCTCCCTC and the downstream primer (SEQ ID No: 2) GGTCTAGATCAGGGCAAGGCGGAGC to amplify the IRES-ZsGreen1 fragment, and use Cla1-Xba1 double enzyme digestion PCR The product was then cloned into the pLVX-Puro vector (Clontech) via the corresponding sites to obtain the pLVX-IRES-ZsGreen1-Puro vector backbone.
[0050] (2) Insert the enhancer Ago2 gene expression cassette CMV-Ago2:
[0051] The pIRESneo-FLAG / HA-Ago2 vector (purchased from Invitrogen, containing the CMV promoter and enhancer Ago2 sequence) was double digested with MluI and EcoRI to obtain a CMV-Ago2 fragment of about 3.3 kb, and the CMV-Ago2 fragment was combined with step (1 )’s pLVX-IRES-ZsGreen1-Puro ve...
Embodiment 2
[0059] Example 2 Construction of shRNA-Ago2 co-expression lentiviral RNAi vector
[0060](1) Prepare the backbone pLVX-IRES-ZsGreen1-Puro for constructing the vector: same as Example 1;
[0061] (2) Insert the enhancer factor Ago2 gene expression cassette CMV-Ago2: same as Example 1;
[0062] (3) Insert shRNA expression promoter
[0063] In this embodiment, the H1 promoter is used, and the primer sequences are SEQ ID No: 7-8 (see Table 2). The vector obtained by referring to the preparation method of Example 1 is: pLVX-H1-CMV-Ago2-IRES-ZsGreen1-Puro.
[0064] (4) Insert the ccdB sequence to obtain the complete shRNA-Ago2 co-expression lentiviral RNAi vector:
[0065] As in Example 1, the shRNA-Ago2 co-expression lentiviral RNAi vector whose vector backbone is pLVX-H1-ccdB-CMV-Ago2-IRES-ZsGreen1-Puro was obtained.
Embodiment 3
[0066] Example 3 Construction of shRNA-Ago2 co-expression lentiviral RNAi vector
[0067] Referring to Examples 1 and 2, the present invention can also use the 7SK promoter, the primer sequence is SEQ ID No: 9-10 (see Table 2), and the prepared vector backbone is pLVX-7SK-ccdB-CMV-Ago2-IRES-ZsGreen1 -Puro's shRNA-Ago2 co-expression lentiviral RNAi vector.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com