Nanoparticle formulation
A technology of nanoparticles and preparations, applied in the field of nanoparticle preparations, to achieve the effect of reducing cancer growth rate and body fat deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1: Preparation of liposomal nanoparticles
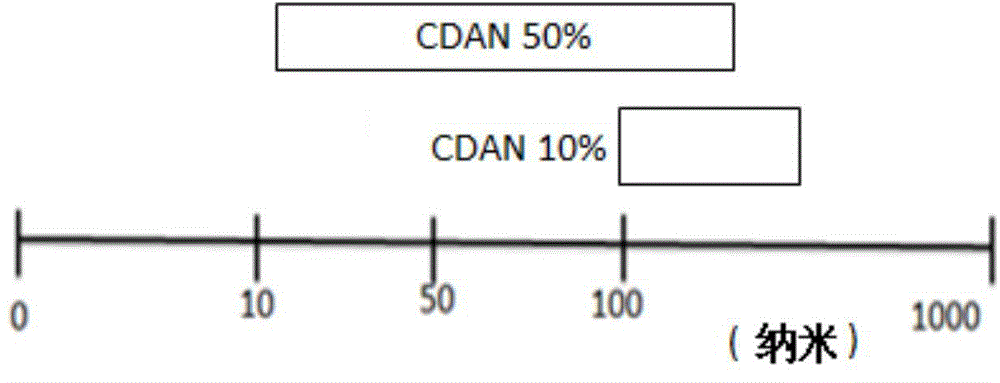
[0082] Take an appropriate volume of N 1 -cholesteryloxy-carbonyl-3,7-diaza-1,9-nonanediamine (CDAN), 1,2-distearoylglycerol-3-phosphatidylcholine (DSPC), cholesterol and 1, Organic solvent (usually CHCl 3 ) stock solution, mixed in a 5 mL round bottom flask at a molar ratio of 32:32:35:1 to form a thin film. The film was then rehydrated with an amount of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (4 mM, NaCl 135 mM, pH 6.5) and sonicated to generate lipid Dispersions, buffered to pH 7, and purified by diafiltration to obtain liposome suspensions at predetermined total lipid concentrations.
Embodiment 2
[0083] Example 2: Preparation of liposomes encapsulating short-chain fatty acids.
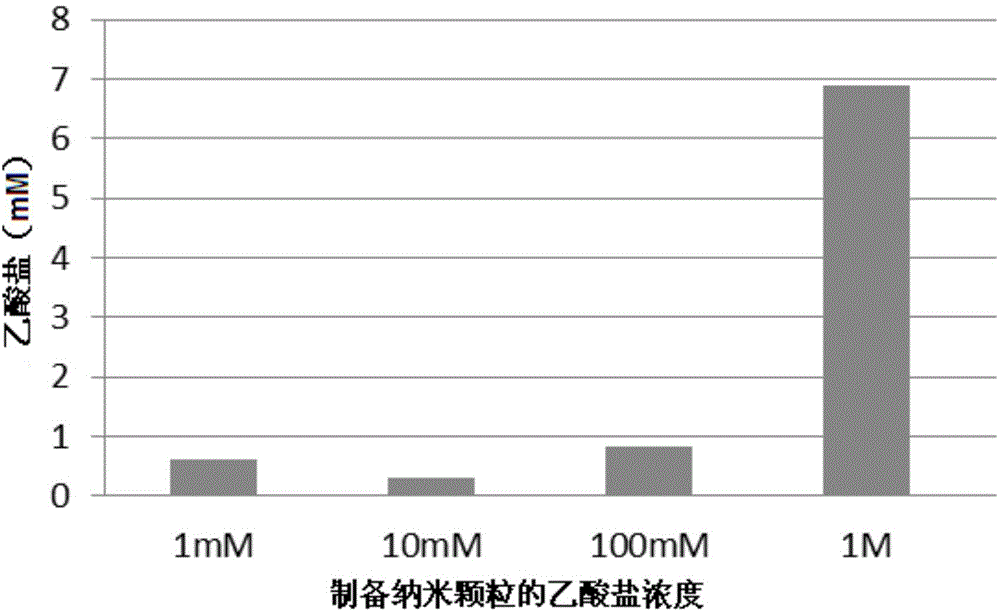
[0084] Take an appropriate volume of N 1 -cholesteryloxy-carbonyl-3,7-diaza-1,9-nonanediamine (CDAN), 1,2-distearoylglycerol-3-phosphatidylcholine (DSPC), cholesterol and 1, Organic solvent (usually CHCl 3 ) stock solution, mixed in a 5 mL round bottom flask at a molar ratio of 32:32:35:1 to form a thin film. The film was then washed with a certain amount of acetic acid solution (1M, CH 3 CO 2 H, pH 2.0) and sonicated to generate a lipid dispersion, buffered to pH 7, and purified by diafiltration to obtain a liposome suspension of predetermined total lipid concentration.
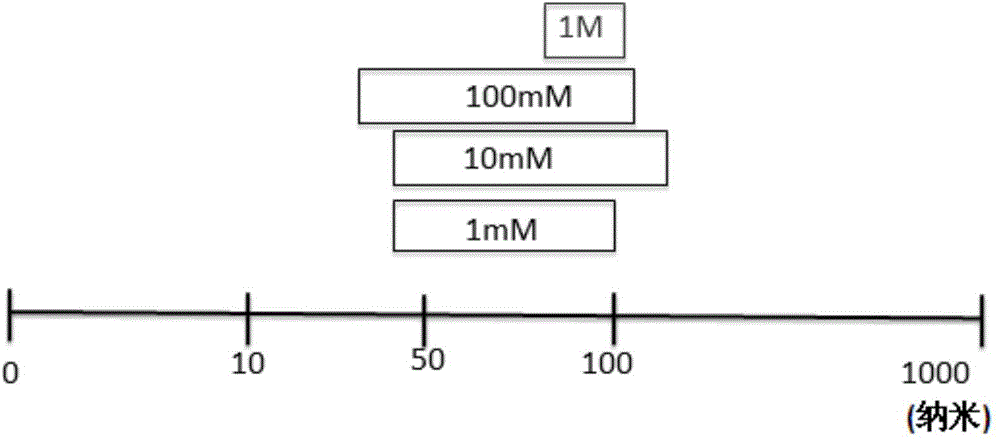
[0085] Liposome-encapsulated acetate nanoparticles (also called "LITA") were prepared in which 1 mL of solution contained approximately 10 11 Nanoparticles / ml. To measure the particle size, such as figure 1 shown.
Embodiment 3
[0086] Example 3: Preparation of fluorescently labeled liposomes.
[0087] Take an appropriate volume of N 1 -cholesteryloxy-carbonyl-3,7-diaza-1,9-nonanediamine (CDAN), 1,2-distearoylglycerol-3-phosphatidylcholine (DSPC), cholesterol, 1, 2-Distearoylglycerol-3-phosphatidylethanolamine-N-(Lissamine Rhodamine B Sulfonyl) (DOPE-rhodamine) and 1,2-Distearoylglycerol-3-phosphatidylethanolamine-N - Organic solvent of methoxy(polyethylene glycol)-2000 (DSPE-PEG2000) (usually CHCl 3 ) stock solution, mixed in a 5 mL round bottom flask at a molar ratio of 32:32:35:1 to form a thin film. The film was then rehydrated with an amount of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (4 mM, NaCl 135 mM, pH 6.5) and sonicated to generate lipid Dispersions, buffered to pH 7, and purified by diafiltration to obtain liposome suspensions at predetermined total lipid concentrations.
[0088] To prepare liposomes containing encapsulated short-chain fatty acids, the lipid compositi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com