Composition containing tenuifoliside and ginsenoside
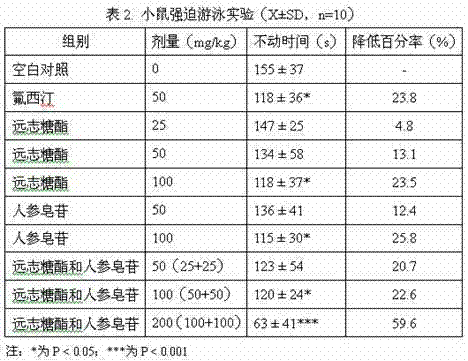
A technology of ginsenosides and polygala sugar esters, which is applied to the composition of polygala sugar esters and ginsenosides to prepare drugs for the treatment and improvement of depression. It can solve the problems of slow onset of action, adverse reactions, and easy recurrence, and achieve enhanced toxicity, The effect of shortening the immobility time of forced swimming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1 : the preparation method of polygala sugar ester and ginsenoside of the present invention
[0012] (1) The effective part of polygala sugar esters used in the present invention can be obtained by referring to the method disclosed in Patent No. 201410215399.5: using polygala medicinal material as raw material, using ethanol solution reflux method to extract, specifically: 70% ethanol with 10 times the quality of polygala medicinal material The solution was used as the extraction solvent, refluxed and extracted 3 times, each time for 1.5 h, the combined extracts were concentrated under reduced pressure until no alcohol smell (60 ℃), and the Polygala extract was obtained. The obtained Polygala extract was purified by D130 macroporous resin. The concentration of the sample solution is 0.25 g / ml containing crude drug, the sample loading speed is 2 Bv / h, and the sample volume is 4 Bv; in the elution process, it is first washed with 5 Bv of water, and the water was...
Embodiment 2
[0014] Example 2 : the maximum tolerated dose experiment of the composition of the present invention
[0015] (1) Materials and methods
[0016] Polygala sugar esters and ginsenosides were obtained by referring to the method in Example 1, and were prepared as a suspension with 0.5% sodium carboxymethylcellulose. Before use, the polygala sugar ester: ginsenoside weight ratios are respectively 0.5:0.5 and 0.11:0.89, and mixed to form a composition.
[0017] The maximum concentration and maximum administration volume of 0.4 mL / 10 g / time gavage was administered twice a day with an interval of 6 hours. The body weight and various symptoms of the mice were observed on the day of administration and within the following two weeks.
[0018] (2) Results:
[0019] On the day and the next day after intragastric administration of the mice, no death occurred in all the administration groups, and no apparent adverse symptoms were observed in the following two weeks. There was no apparen...
Embodiment 3
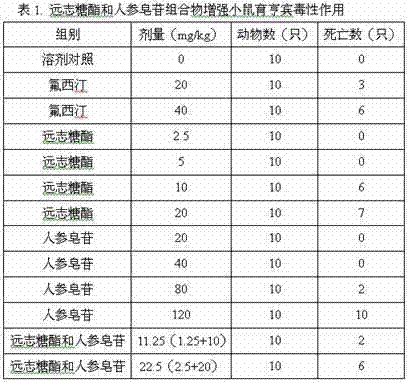
[0022] Example 3 : Mouse Yohimbine Toxicity Enhancement Experiment
[0023] (1) Materials and methods:
[0024] Polygala sugar esters and ginsenosides were obtained by referring to the method in Example 1, according to the weight ratio of polygala sugar esters: ginsenosides 0.11:0.89.
[0025] Yohimbine Hydrochloride: Aladdin Chemistry Co., Ltd. Batch No.: 29107. Normal saline: Hangzhou Jiutian Animal Health Products Co., Ltd. Batch number: 2014071901.
[0026] Male ICR mice, clean grade, weighing 18-24 g, were given standard pelleted feed and pure drinking water during the feeding period. Set solvent control group, fluoxetine positive drug control group (20 and 40 mg / kg), polygala sugar ester group (2.5, 5 and 10 mg / kg), ginsenoside group (20, 40 and 80 mg / kg), Polygala sugar ester and ginsenoside composition group (11.25 and 22.5 mg / kg), administered by intraperitoneal injection. After administration of the test drug or solvent, a sublethal dose of yohimbine (25 mg / kg)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com