Production method of N-acetyl-L-cysteine
A technology of cysteine and production method, applied in sulfide preparation, organic chemistry and other directions, can solve the problems of affecting product quality, increasing product impurity content and production cost, and high content, and achieves the effect of reducing impurity content and production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] A production method of N-acetyl-L-cysteine, comprising the following steps:
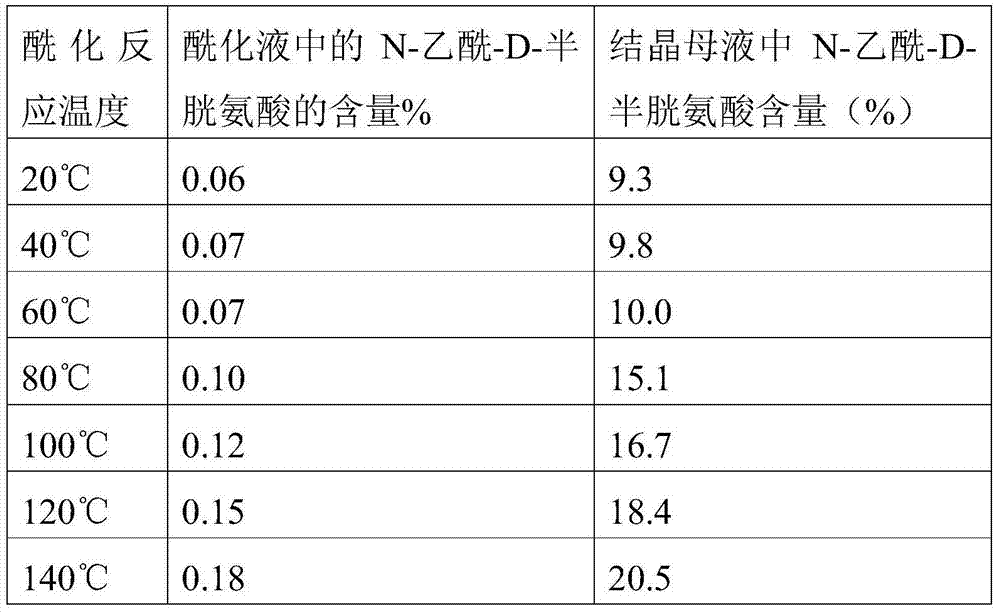
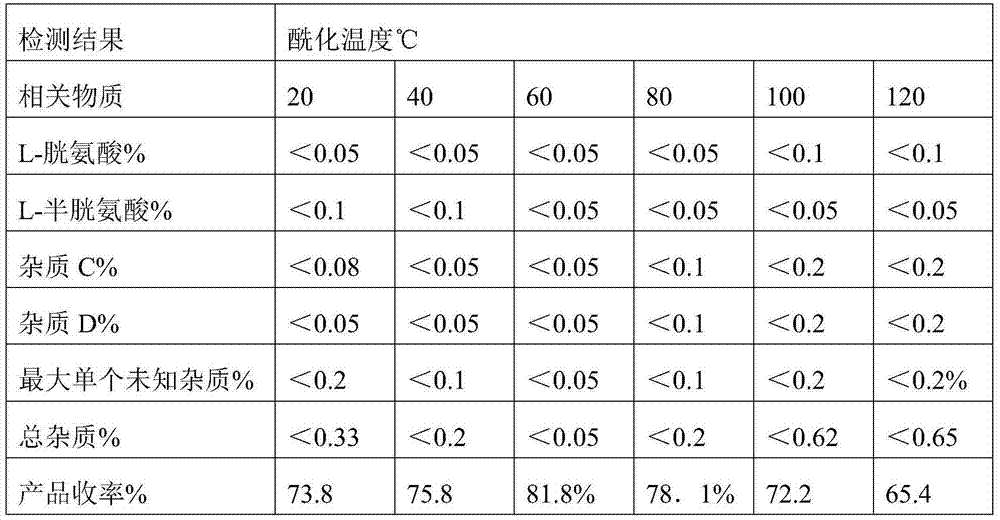
[0016] 1) Add 1200Kg weight content to the reactor and be the aqueous solution of 50% L-cysteine hydrochloride monohydrate (equivalent to 600kg of L-cysteine hydrochloride monohydrate), then add 20% Sodium hydroxide solution, adjust the pH value to 10, start to slowly add acetic anhydride and carry out acylation reaction under normal pressure, when the temperature of the reaction solution rises to 60°C, circulate the reaction solution through the heat exchange device to make the reaction The temperature of the reaction solution in the kettle was maintained at 60°C until the acetic anhydride was added (a total of 300Kg was added, which took 1.5 hours), and then the insulation was continued for 1 hour;
[0017] 2) adding hydrochloric acid to the reaction solution, adjusting the pH to 2, concentrating in vacuo, cooling to crystallize, and centrifuging to collect the crystals to obtain crude N...
Embodiment 2
[0020] A production method of N-acetyl-L-cysteine, comprising the following steps:
[0021] 1) Adding 1090Kg weight content in the reactor is an aqueous solution of 55% L-cysteine hydrochloride monohydrate (equivalent to 600kg of cysteine hydrochloride monohydrate), then adding sodium hydroxide solution, Adjust the pH value to 8, start to slowly add acetic anhydride and carry out the acylation reaction under normal pressure, when the temperature of the reaction solution rises to 20°C, circulate the reaction solution through the heat exchange device to make the reaction solution in the reactor The temperature was kept at 50°C until the acetic anhydride was added (a total of 300Kg was added, which took 1.5 hours), and then kept for 2 hours;
[0022] 2) adding hydrochloric acid to the reaction solution, adjusting the pH to 2.5, concentrating in vacuo, cooling to crystallize, and centrifuging to collect the crystals to obtain crude N-acetyl-L-cysteine;
[0023] 3) Dissolve th...
Embodiment 3
[0025] A production method of N-acetyl-L-cysteine, comprising the following steps:
[0026] 1) Adding 1000Kg weight content to the reactor is an aqueous solution of 60% L-cysteine hydrochloride monohydrate (equivalent to 600kg of cysteine hydrochloride monohydrate), then adding sodium hydroxide solution, Adjust the pH value to 11, start slowly adding acetic anhydride and carry out the acylation reaction under normal pressure, when the temperature of the reaction solution rises to 90°C, circulate the reaction solution through the heat exchange device to make the reaction solution in the reactor The temperature was kept at 70°C until the acetic anhydride was added (a total of 300Kg was added, which took 1 hour), and then kept for 0.5 hours;
[0027] 2) adding hydrochloric acid to the reaction solution, adjusting the pH to 2, concentrating in vacuo, cooling to crystallize, and centrifuging to collect the crystals to obtain crude N-acetyl-L-cysteine;
[0028] 3) Dissolve the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com