Purification method of recombinant herpes simplex virus
A technology of herpes simplex virus and purification method, which is applied in the field of purification of recombinant herpes simplex virus, can solve the problems of high price, high cost, and difficult promotion, and achieve the effect of no virus damage, low cost, and good purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
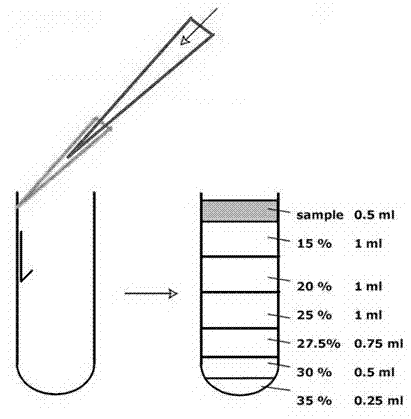
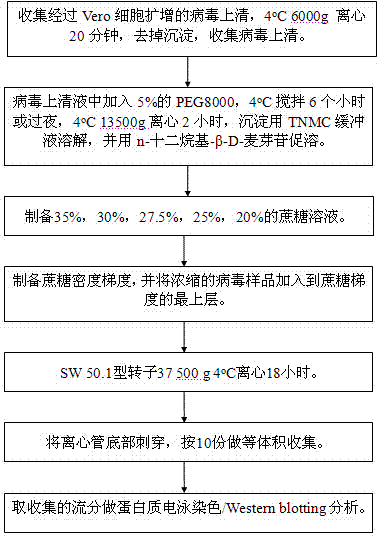
[0062] I. Virus Proliferation
[0063] Use a 10 mL sterile pipette to draw 6 mL of PBS from Vero cells with a cell density of 80%-90% to wash the cells three times; o C adsorption for 1 hour; add virus growth solution and maintenance solution to the cell bottle, and culture it in a 37 ℃ constant temperature incubator until 75-100% of the cells have CPE lesions. Harvest. Before harvesting, the cells can be placed in a -70 ℃ refrigerator and frozen Melt 2 to 3 times, 4 o Centrifuge at 6000g for 20 minutes, remove the precipitate, and collect 50 mL of virus supernatant.
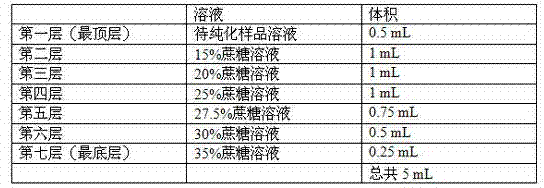
[0064] II. Virus Concentration
[0065] Add 5% PEG8000 to the supernatant, 4 o C stirring for 6 hours or overnight, 4 o Centrifuge at 13500g for 2 hours; dissolve the precipitate with TNMC buffer, and add n-dodecyl-β-D-maltoside to the solution to a concentration of 20mM to promote the dissolution of the precipitate, and finally make it centrifuged to purify the virus sample The final volume is 1% of that b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com