Variable region gene of full human monoclonal antibody specific to pro-protein convertase subtilisin/kexin 9 (PCSK9) and application thereof
A monoclonal antibody, fully human technology, applied in applications, antibodies, genetic engineering, etc., can solve problems such as abnormal liver enzymes, rhabdomyolysis, statin intolerance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Construction of Human Monoclonal Antibody Phage Display Library
[0037] The present invention first constructs a natural human Fab phage display library according to the report of Hans J.W.de Haard et al. 10 , the host is Escherichia coli TG1.
Embodiment 2
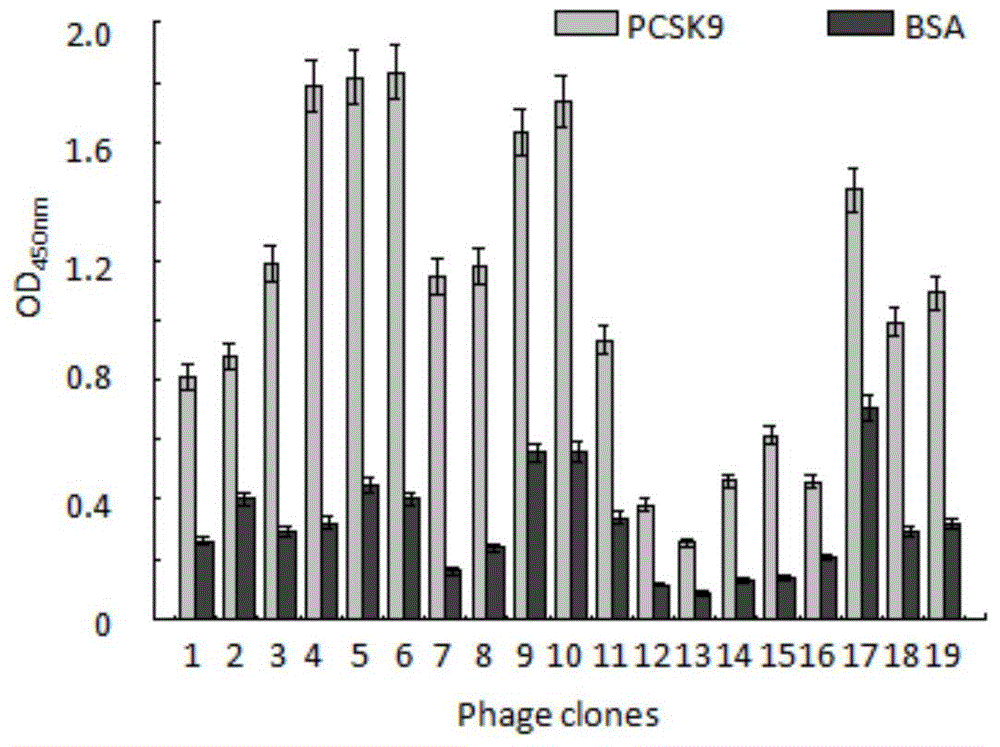
[0038] Example 2, screening the Fab phage library to obtain a positive monoclonal anti-human PCSK9
[0039] 1. Dilute PCSK9 protein (purchased from GenScript Biotechnology Co., Ltd.) with coating buffer (PBS) to 100 μg / ml; take 2 ml into the immunotube, and coat overnight at 4°C; the next day, discard the supernatant, Wash the tube 3 times with PBS; 2% MPBS (PBS containing 2% skimmed milk powder), block at room temperature for 2h; discard the blocking solution; phage antibody library (10 12 ~10 13 pfu) was suspended in 2ml 2% MPBS, placed on a turntable and repeatedly turned over, and sealed at room temperature for 1 hour; the blocked phage antibody library was added to a PCSK9-coated immune tube, placed on a turntable and turned over repeatedly for 30 minutes, and then allowed to stand at room temperature 90min; wash the tube 20 times with PBS containing 0.1% Tween-20, then wash the tube 20 times with PBS; add 1ml of 100mM triethylamine, place on a turntable and incubate for...
Embodiment 3
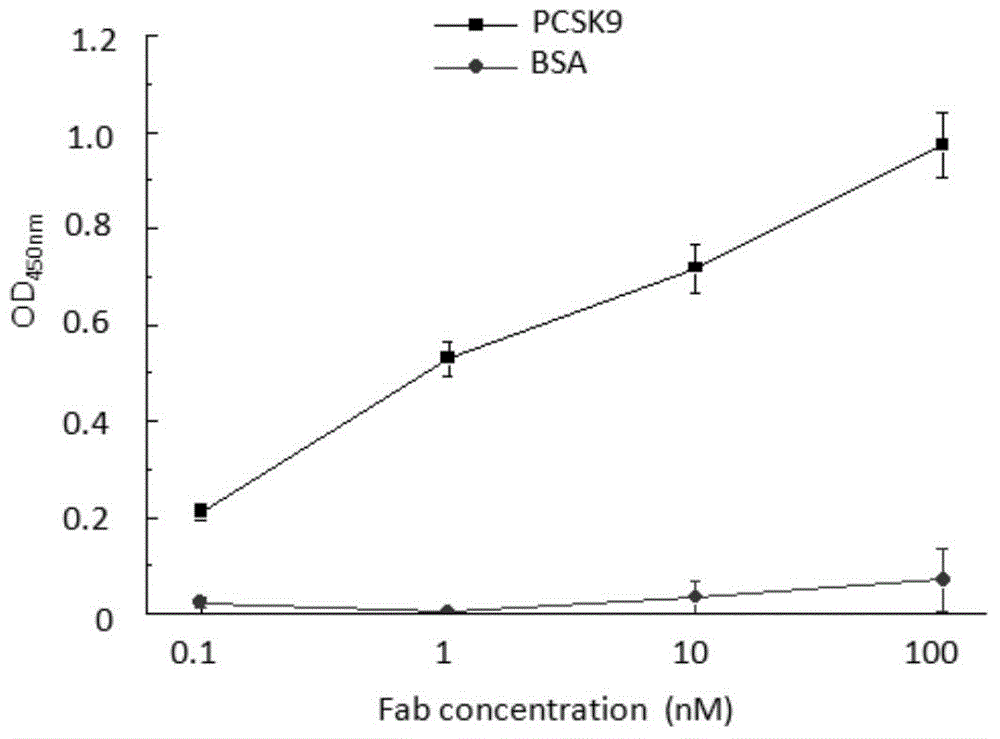
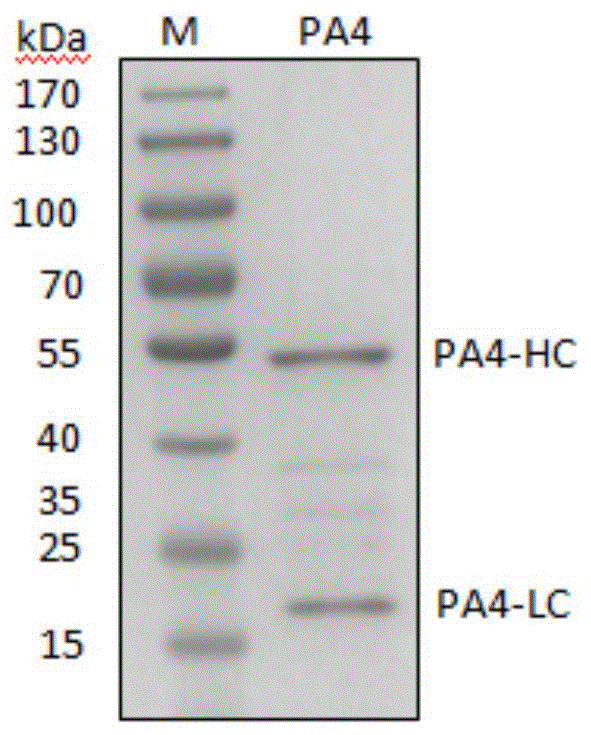
[0043] Example 3, Expression and Purification of Positive Monoclonal PA4 Antibody Fab Fragment
[0044] 1. Select the PA4-infected TG1 strain for culture, use the plasmid mini-extraction kit to extract, and collect the PA4 phagemid vector. According to the report of Schoonbroodt S et al. (Nucleic Acids Research, Schoonbroodt S, 2005, 33, e81), the phagemid vector was digested with MluI restriction endonuclease to remove Gene III.
[0045] 2. Transform the obtained GeneIII excised plasmid into Escherichia coli TG1, 2YTA medium, culture to logarithmic phase at 37°C, add 1mM IPTG, induce expression overnight at 30°C.
[0046] 3. Collect the supernatant by centrifugation, and use Protein A to purify to obtain PA4Fab protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com