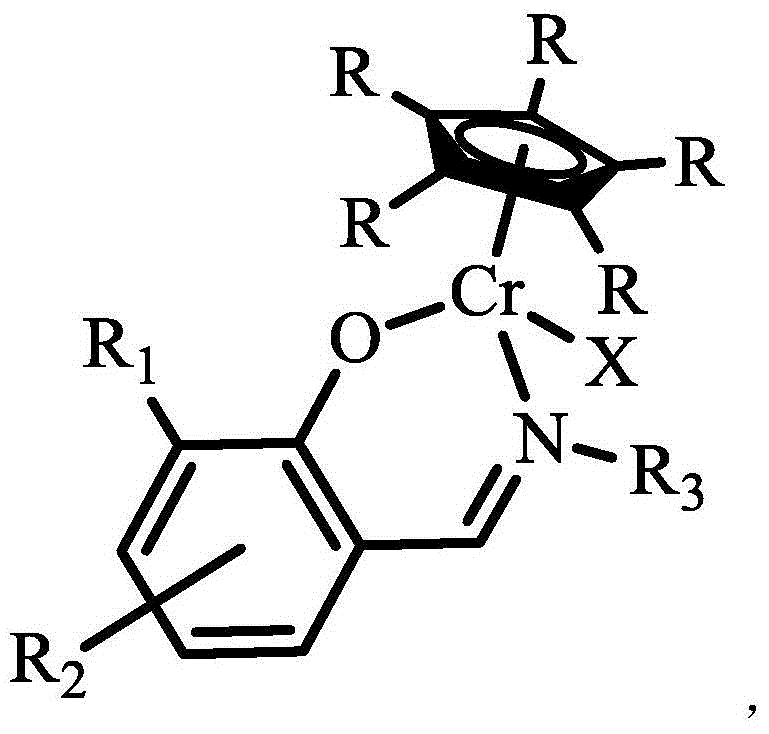
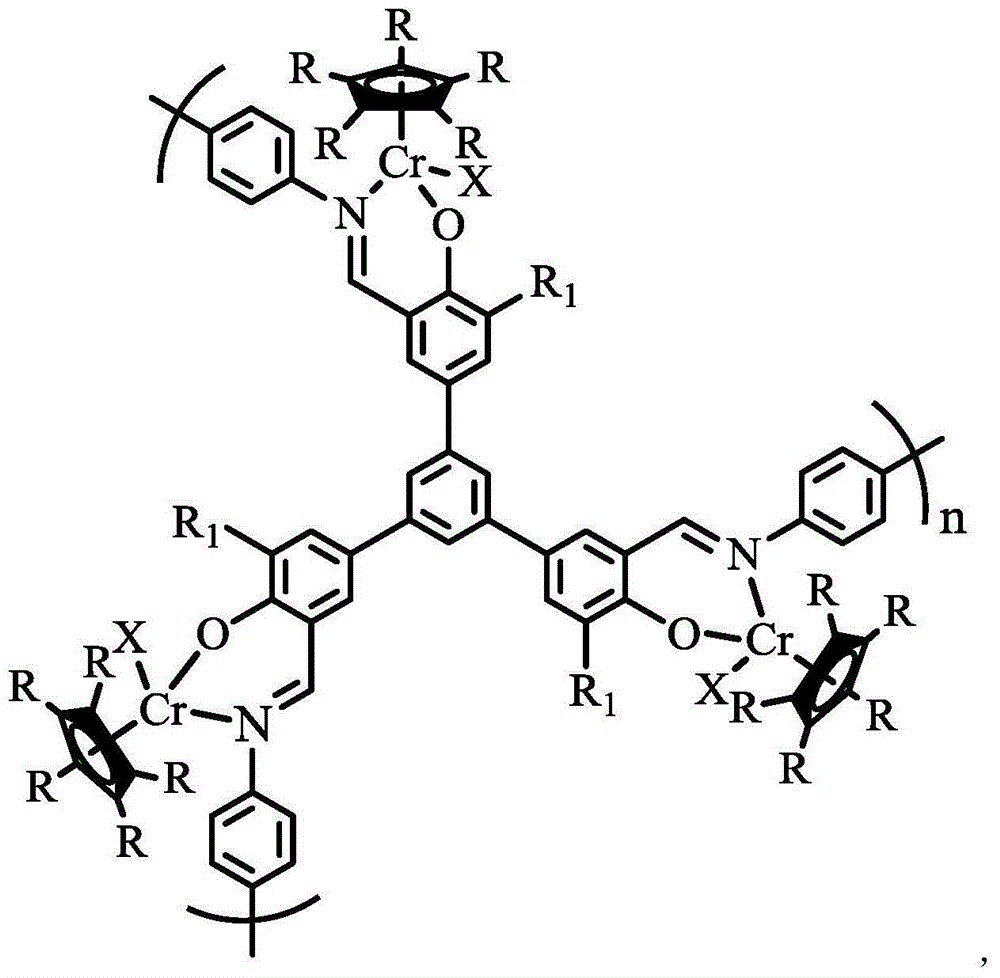
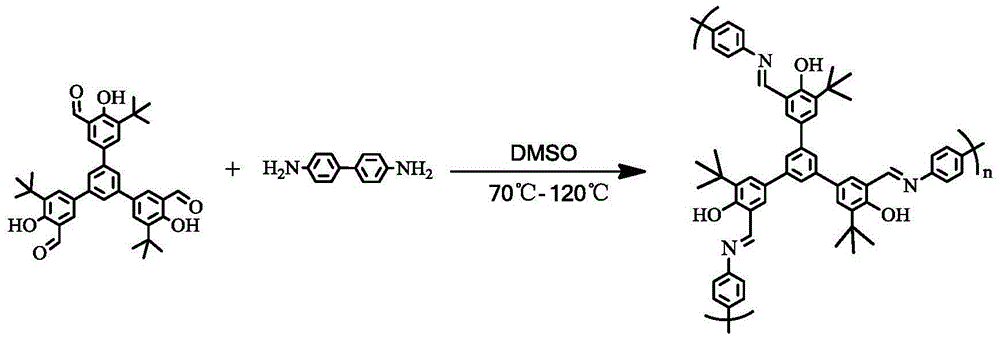
Organic porous material after-supported single cyclopentadienyl chromium catalyst and application thereof
A porous material and post-loading technology, which is applied in the field of organic porous coordination polymer materials, can solve the problems of introducing ash impurities and decreasing catalytic activity, and achieve the effects of high catalytic activity, good stability and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] 400 mg (0.66 mmol) of 1,3,5-tris(3-tert-butyl-4-hydroxy-5-formylphenyl)benzene, 182 mg (0.99 mmol) of benzidine and 15 mL of DMSO were added to a 25 ml flask. The reaction mixture was successively reacted in an oil bath at 70°C for 12 hours, at 100°C for one day, and at 120°C for two days. The reaction solution was filtered, and the filter cake was washed with THF, DMF, and CH3OH. The solid was extracted with THF and CH3OH for 6 hours, and then vacuum-dried at 120°C. The obtained orange-yellow solid powder was recorded as Ligand 1, with a yield of 492.6 mg and a yield of 90.1%.
[0038] The synthesis of 1,3,5-tris(3-tert-butyl-4-hydroxy-5-formylphenyl)benzene reference Liu H., Wang M., Wang Y., etal.Synthetic Commun., 2010, 40:1074-1081.
Embodiment 2
[0040]
[0041] 400 mg (0.64 mmol) of 1,3,5-tris(3-phenyl-4-hydroxy-5-formylphenyl)benzene, 182 mg (0.99 mmol) of benzidine and 15 mL of DMSO were added to a 25 ml flask. The reaction mixture was reacted successively in an oil bath at 70°C for 12 hours, at 100°C for one day, and at 120°C for two days. The reaction solution was filtered and washed with THF, DMF, CH 3 OH to wash the filter cake, and the solid was washed with THF, CH 3 OH was extracted by Soxhlet for 6 hours, and then vacuum-dried at 120 °C. The obtained orange-yellow solid powder was recorded as Ligand 2, with a yield of 462.1 mg and a yield of 84.7%.
[0042] The synthesis of 1,3,5-tris(3-phenyl-4-hydroxy-5-formylphenyl)benzene refers to the literature Liu H., Wang M., Wang Y., etal.Synthetic Commun.,2010,40 :1074-1081.
Embodiment 3
[0044]
[0045] Under a nitrogen atmosphere, dissolve 0.16 g of Ligand 1 (containing 0.58 mmol-OH) prepared in Example 1 in 20 mL of anhydrous tetrahydrofuran, add 0.58 mmol of n-butyllithium at -78 ° C, and stir at room temperature for 2 Hour. Add 10 mL tetrahydrofuran solution of 58 mmol / L pentamethyl-substituted monochromocene at -78°C, warm to room temperature, stir for 20 hours, and remove the solvent by filtration. The solid product was washed with dry tetrahydrofuran and dried under vacuum at 140°C for 4 hours. That is, 0.200 g of the organic porous material was obtained to support the metallocene chromium catalyst, which was recorded as complex 1, and the yield was 69.4%. Elemental analysis of its composition: C 73.59%, H 7.62%, N 5.32%. The Cr content measured by ICP was 3.34%. The specific surface area of complex 1 measured by the specific surface area analyzer is 504m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com