Indapamide slow-release hypertension pill and preparation method thereof
A technology for indapamide and antihypertensive tablets, which is applied in the field of indapamide sustained-release antihypertensive tablets and its preparation, can solve the problem of damage to the sustained-release function of hypromellose, drugs that do not meet the quality standards for regularization, and product quality Instability and other problems, to avoid the sudden release of the drug effect, reduce the economic burden, and achieve the effect of gentle and long-lasting antihypertensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
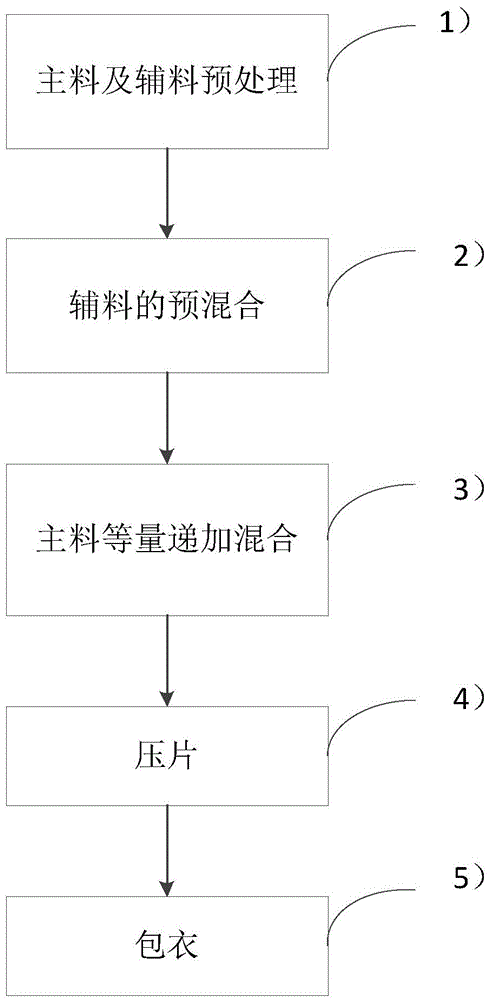
Image
Examples
Embodiment 1
[0026] Based on the above, the framework materials are selected as hypromellose RT4000 and hypromellose RT5, the disintegrating material is microcrystalline cellulose, the diluent is lactose, the lubricant is magnesium stearate, and the weight of each component The percentages are: indapamide, 0.75 parts; hypromellose RT4000, 25 parts; hypromellose RT5, 23.75 parts; microcrystalline cellulose, 10 parts; lactose, 40 parts; magnesium stearate, 0.5 Copies.
[0027] Hypromellose RT4000 and hypromellose RT5 are used as the backbone material. RT4000 / 5 represents its viscosity model. It contains two main functional groups, namely methoxy and hydroxypropoxy. The oxy group is a hydrophobic group, which is resistant to corrosion and has a slow release effect. The hydroxypropoxy group is a hydrophilic group that quickly forms a gel layer after contact with water, which can inhibit the burst release of the drug. Lactose plays a role of diluting in the prescription, and has good compressibil...
Embodiment 2
[0029] Based on the above, the framework materials used are hypromellose RT4000 and hypromellose RT5, the disintegrating material is microcrystalline cellulose, the diluent is lactose, the lubricant is magnesium stearate, and the weight of each component The percentages are: indapamide, 0.75 parts; hypromellose RT4000, 28.75 parts; hypromellose RT5, 15 parts; microcrystalline cellulose, 5 parts; lactose, 50 parts; magnesium stearate, 0.5 Copies.
Embodiment 3
[0031] Based on the above, the framework materials used are hypromellose RT4000 and hypromellose RT5, the disintegrating material is microcrystalline cellulose, the diluent is lactose, the lubricant is magnesium stearate, and the weight of each component The percentages are: indapamide, 0.75 parts; hypromellose RT4000, 30 parts; hypromellose RT5, 25 parts; microcrystalline cellulose, 8 parts; lactose, 35.75 parts; magnesium stearate, 0.5 Copies.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com