Radioactive halogen marked micromolecule cyclic peptide, composition and application of radioactive halogen marked micromolecule cyclic peptide
A radioactive, small molecule technology, applied in the field of medicine, can solve the problems of difficult deep tissue localization and expression, lack of clinical application prospects, short fluorescence imaging distance, etc., and achieve high thermodynamic and dynamic stability, strong penetrability, good The effect of visualization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
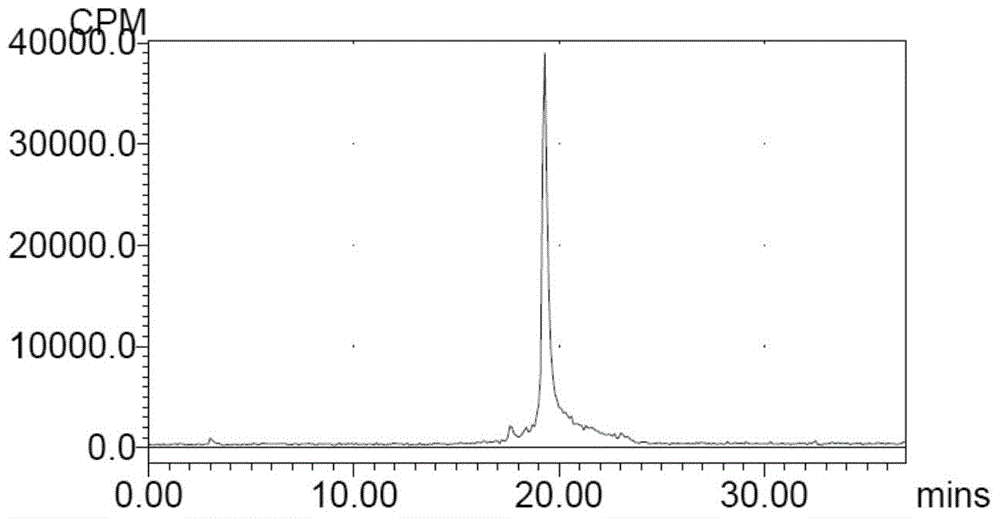
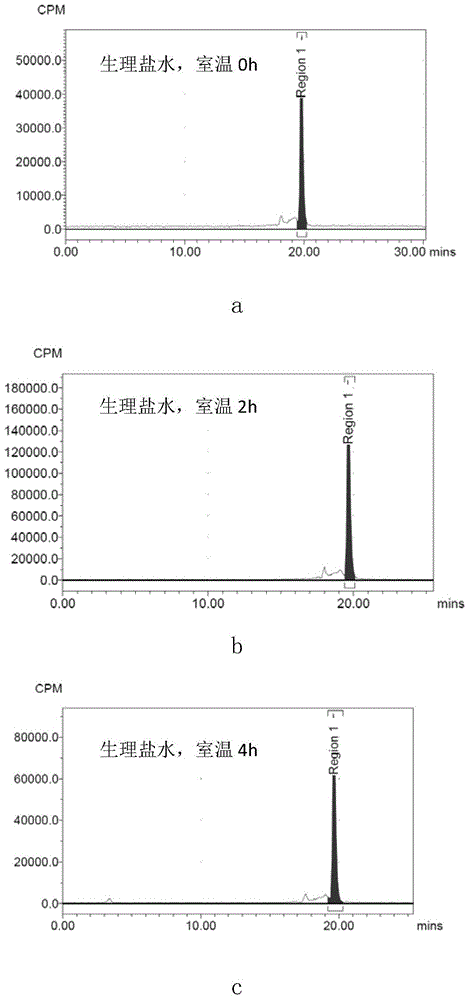
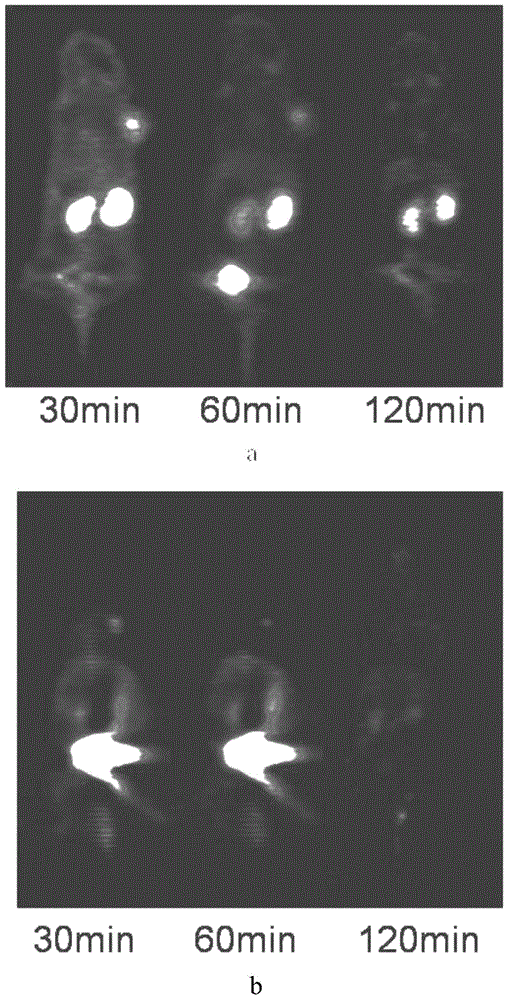
[0031] The radioactive halogen-labeled small molecule cyclic peptides, compositions and applications of the present invention will be further described in conjunction with the accompanying drawings.
[0032]
[0033] In this example, the selected chelating ligand is NOTA, and the metal complex containing radioactive halogen is Al 18 F complex, the small molecule cyclic peptide is c(KAHWGFTLD)NH2, which is C6. In this example, c(KAHWGFTLD)NH-NOTA (C6-NOTA) was selected for labeling. C6-NOTA was synthesized by Hangzhou Zhongpei Biochemical Co., Ltd. by solid-phase synthesis.
[0034] 1. C6-NOTA-Al- 18 Preparation of F
[0035] Step 1: Using Nuclear Reactions on a Cyclotron 18 O(P,n) 18 F made [ 18 F]F - ;
[0036] Step 2: Add sterilized water for injection to C6-NOTA to dissolve, and prepare a 1 mg / ml solution;
[0037] Step 3: Take 30 μl of C6-NOTA sterile aqueous solution for injection, add 400 μl of acetonitrile, 6 μl of 2 mmol / L aluminum chloride aqueous solution, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com