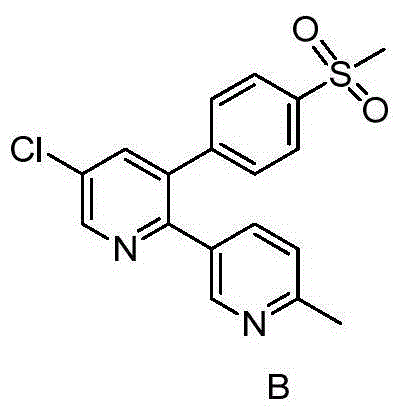
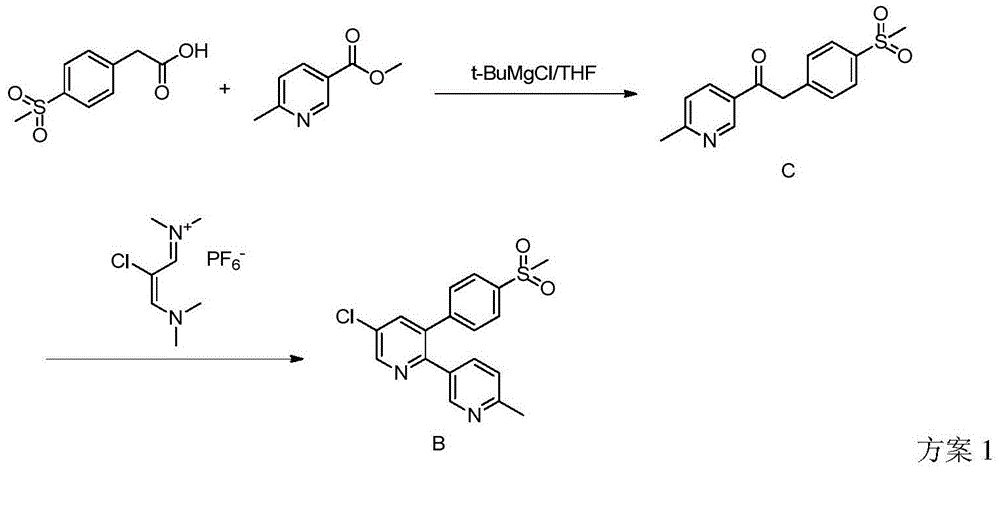
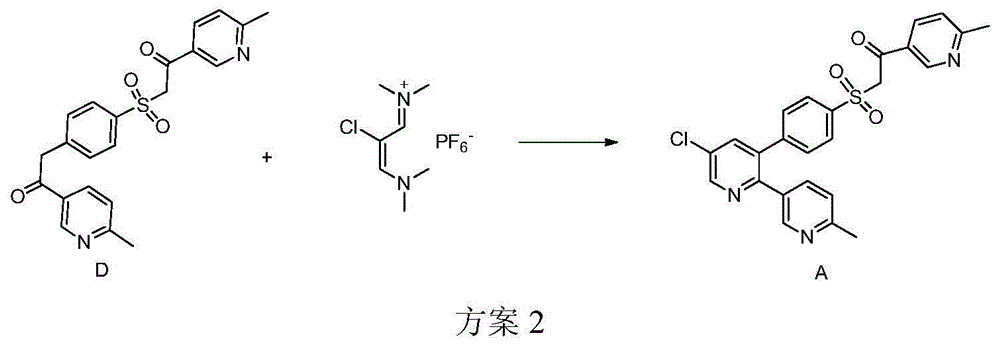
Preparation method of reference substance for synthesizing etoricoxib
A technology of etoricoxib and reference substance, applied in the field of drug synthesis, can solve the problems of high labor cost, difficult preparation method, etc., and achieve the effects of simple synthesis method and easy-to-obtain reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Mix 1.44g (4.0mmol) etoricoxib, 0.60g (4.0mmol) methyl 6-methylnicotinate, 0.22g (4.0mmol) sodium methoxide and 6ml dichloromethane, and react at room temperature for 10h. After the reaction was monitored by TLC, 15ml of water and 15ml of dichloromethane were added, separated, the aqueous phase was extracted with dichloromethane (15ml×2), combined with dichloromethane, washed twice with saturated brine, separated, and the organic phase was used Dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, and purify by column chromatography to obtain 1.10 g of white solid, namely Compound A, with a yield of 57.9%, m.p.187-189°C. EIMS m / z(%):478.0(M + ,100).
[0021] 1 H NMR (500MHz, CDCl 3 ): δ2.588(s,3H),2.658(s,3H),4.739(s,2H),7.126(d,J=8.5Hz,1H),7.316(d,J=8.5Hz,1H),7.390 (d, J=8.5Hz, 2H), 7.498(s, 1H), 7.762(d, J=2.5Hz, 1H), 7.858(d, J=8Hz, 2H), 8.161(m, 1H), 8.491( d,J=2.0Hz,1H),8.732(d,J=2.5Hz,1H),9.023(d,J=2.0Hz,1H). 13 C NMR (125MHz, CDCl 3...
Embodiment 2
[0023] Mix 1.44g (4.0mmol) etoricoxib, 0.73g (4.8mmol) methyl 6-methylnicotinate, 0.19g (4.8mmol) sodium hydride (60% content) and 12ml tetrahydrofuran, heat to reflux for 4h . After the reaction was detected by TLC, slowly add 15ml of water and 15ml of dichloromethane, separate the layers, extract the aqueous phase with dichloromethane (15ml×2), combine the dichloromethane, wash twice with saturated saline, separate the layers, and organic phase After drying with anhydrous sodium sulfate, the solvent was removed under reduced pressure, and purified by column chromatography to obtain 1.01 g of white solid, namely compound A, with a yield of 52.9%.
Embodiment 3
[0025] Mix 2.88g (8.0mmol) etoricoxib, 1.81g (12.0mmol) methyl 6-methylnicotinate, 1.35g (12.0mmol) potassium tert-butoxide and 25ml tetrahydrofuran, and heat to reflux for 4h. After the reaction was detected by TLC, slowly add 30ml of water and 35ml of dichloromethane, separate the liquids, extract the aqueous phase with dichloromethane (35ml×2), combine the dichloromethane, wash twice with saturated saline, separate the liquids, and organic phase After drying with anhydrous sodium sulfate, the solvent was removed under reduced pressure, and purified by column chromatography to obtain 2.32 g of white solid, ie Compound A, with a yield of 60.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com